phlogiston
turning alchemy into chemistry
The phlogiston theory
Phlogiston theory postulated the existence of a fire-like element called phlogiston contained within combustible bodies and released during combustion.
Empedocles formulated the classical theory that there were four elements—water, earth, fire, and air—and Aristotle reinforced this idea by characterising them as moist, dry, hot, and cold. Fire was thus thought of as a substance, and burning was seen as a process of decomposition that applied only to compounds. Experience had shown that burning was not always accompanied by a loss of material, and a better theory was needed to account for this.
Phlogiston
iMPORTANCE
The theory of phlogiston led to the discovery of oxygen and is seen as the transition between alchemy and chemistry.

Phlogiston
ETYMOLOGY
The name comes from the Ancient Greek φλογιστόν phlogistón (burning up), from φλόξ phlóx (flame).

Phlogiston
Challenge
Phlogiston theory was challenged by the concomitant weight increase and was abandoned before the end of the 18th century.
Phlogiston accounted for combustion via a process that was the inverse of that of the oxygen theory. Phlogisticated substances contain phlogiston and they dephlogisticate when burned, releasing stored phlogiston which is absorbed by the air. Growing plants then absorb this phlogiston, which is why air does not spontaneously combust and also why plant matter burns as well as it does.
"In general, substances that burned in the air were said to be rich in phlogiston; the fact that combustion soon ceased in an enclosed space was taken as clear-cut evidence that air had the capacity to absorb only a finite amount of phlogiston. When the air had become completely phlogisticated it would no longer serve to support the combustion of any material, nor would a metal heated in it yield a calx; nor could phlogisticated air support life. Breathing was thought to take phlogiston out of the body."
Joseph Black's Scottish student Daniel Rutherford discovered nitrogen in 1772, and the pair used the theory to explain his results. The residue of air left after burning, in fact, a mixture of nitrogen and carbon dioxide, was sometimes referred to as phlogisticated air, having taken up all of the phlogiston. Conversely, when Joseph Priestley discovered oxygen, he believed it to be dephlogisticated air, capable of combining with more phlogiston and thus supporting combustion for longer than ordinary air.
During the eighteenth century, as it became clear that metals gained weight after they were oxidized, phlogiston was increasingly regarded as a principle rather than a material substance. By the end of the eighteenth century, for the few chemists who still used the term phlogiston, the concept was linked to hydrogen.
BECHER
His idea that combustible substances contain an ignitable matter, which he called terra pinguis, would become the phlogiston theory
Johann Joachim Becher (1635 – 1682) was a German physician, alchemist, precursor of chemistry, scholar, polymath and adventurer, best known for his development of the phlogiston theory of combustion, and his advancement of Austrian cameralism.
In 1667, Johann Joachim Becher published his book, Physica subterranea, which contained the first instance of what would become the phlogiston theory. In his book, Becher eliminated fire and air from the classical element model and replaced them with three forms of the earth: terra lapidea, terra fluida, and terra pinguis. Terra pinguis was the element that imparted oily, sulphurous, or combustible properties. Becher believed that terra pinguis was a key feature of combustion and was released when combustible substances were burned. Georg Ernst Stahl was Becher's student and further developed the phlogiston theory.
William Cullen considered Becher as a chemist of first importance and Physica Subterranea as the most considerable of Bechers writings.
WORKS
- Works by or about Johann Joachim Becher at Internet Archive
- Johann Joachim Becher-Preises (in German)
- Engines of our Ingenuity: Johann Joachim Becker at uh.edu
- Texts on Wikisource:
- Joh. Joach. Becheri … Opuscula chymica rariora, addita nova praefatione ac indice locupletissimo multisque figuris aeneis illustrata a Friderico Roth-Sholtzio, Siles – full digital facsimile from Linda Hall Library
“Chemistry as an earnest and respectable science is often said to date from 1661, when Robert Boyle of Oxford published The Sceptical Chymist — the first work to distinguish between chemists and alchemists — but it was a slow and often erratic transition. Into the eighteenth century scholars could feel oddly comfortable in both camps — like the German Johann Becher, who produced sober and unexceptionable work on mineralogy called Physica Subterranea, but who also was certain that, given the right materials, he could make himself invisible.”
-Bill Bryson, A Short History of Nearly Everything
STAHL
proposed that metals were made of calx, or ash, and phlogiston and that once a metal is heated, the phlogiston leaves only the calx within the substance
Georg Ernst Stahl (1659–1734) was a German chemist, physician, philosopher and supporter of vitalism.
In 1703, Stahl proposed a variant of the theory in which he renamed Becher's terra pinguis to phlogiston, and it was in this form that the theory probably had its greatest influence.
The term 'phlogiston' itself was not something that Stahl invented. There is evidence that word was used as early as 1606, and in a way that was very similar to what Stahl was using it for. The term was derived from a Greek word meaning inflame.
Stahl's first definition of phlogiston first appeared in his Zymotechnia fundamentalis, published in 1697. His most quoted definition was found in the treatise on chemistry entitled Fundamenta chymiae in 1723. According to Stahl, phlogiston was a substance that was not able to be put into a bottle but could be transferred nonetheless.
To Stahl, wood was just a combination of ash and phlogiston, and making a metal was as simple as getting a metal calx and adding phlogiston. Soot was almost pure phlogiston, which is why heating it with a metallic calx transforms the calx into the metal and Stahl attempted to prove that the phlogiston in soot and sulphur were identical by converting sulphates to liver of sulphur using charcoal. He did not account for the increase in weight on combustion of tin and lead that were known at the time but those who followed his school of thought worked on this problem.
The following paragraph describes Stahl's view of phlogiston:
"To Stahl, metals were compounds containing phlogiston in combination with metallic oxides (calces); when ignited, the phlogiston was freed from the metal leaving the oxide behind. When the oxide was heated with a substance rich in phlogiston, such as charcoal, the calx again took up phlogiston and regenerated the metal. Phlogiston was a definite substance, the same in all its combinations."
Leicester, Henry M.; Klickstein, Herbert S. (1965). A Source Book in Chemistry. Cambridge, Massachusetts: Harvard University Press.
Phlogiston theory did not have any experimental basis before Stahl worked with metals and various other substances in order separate phlogiston from them.
Stahl was able to make phlogiston theory applicable to chemistry as it was one of the first unifying theories in the discipline. Stahl's theory of phlogiston is seen as the transition between alchemy and chemistry. This theory was later replaced by Lavoisier's theory of oxidation and caloric theory.
Until the late 18th century his works on phlogiston were an accepted explanation for chemical processes.
WORKS
- Zymotechnia fundamentalis (1697)
- De lumbricis terrestribus eorumque usu medico (in Latin). Halle: Christian Henckel. 1698.
- Disquisitio de mechanismi et organismi diversitate (1706)
- Paraenesis, ad aliena a medica doctrine arcendum (1706)
- De vera diversitate corporis mixti et vivi (1706)
- Theoria medica vera (1708)
- De lapide manati (in Latin). Halle: Christian Henckel. 1710.
- Georgii Ernesti Stahlii opusculum chymico-physico-medicum : seu schediasmatum, a pluribus annis variis occasionibus in publicum emissorum nunc quadantenus etiam auctorum et deficientibus passim exemplaribus in unum volumen iam collectorum, fasciculus publicae luci redditus / Praemißa praefationis loco authoris epistola ad Michaelem Alberti (1715) Digital edition by the University and State Library Düsseldorf
- Specimen Beccherianum (1718)
- Philosophical Principles of Universal Chemistry (1730), Peter Shaw, translator, from Open Library.
- Experimenta, observationes, animadversiones, 300. numero, chymicae et physicae (in Latin). Berlin: Ambrosius Haude. 1731.
- Materia medica : das ist: Zubereitung, Krafft und Würckung, derer sonderlich durch chymische Kunst erfundenen Artzneyen (1744), Vol. 1&2 Digital edition by the University and State Library Düsseldorf
- Fundamenta chymiae (in Latin). Vol. 3. Nürnberg: Wolfgang Moritz Endter, Erben & Julius Arnold Engelbrecht, Witwe. 1747.
- Fundamenta chymiae (in Latin). Vol. 2. Nürnberg: Wolfgang Moritz Endter, Erben & Julius Arnold Engelbrecht, Witwe. 1746.
- The Leibniz-Stahl Controversy (2016), transl. and edited by F. Duchesneau and J. H. Smith, Yale UP (536 pp.)
Juncker
CONCLUDED phlogiston has the property of levity or that it makes the compound it is in much lighter than it would be without the phlogiston
Johann Juncker (1679 – 1759 ) was a German physician and chemist. Juncker was a leader in the Pietist reform movement as it applied to medicine. He directed the Francke Foundations and initiated approaches to medical practice, charitable treatment, and education at the University of Halle that influenced others internationally. He was a staunch proponent of Georg Ernst Stahl and helped to more clearly present Stahl's phlogiston theory of combustion.
When reading Stahl's work, he assumed that phlogiston was in fact very material. He, therefore, came to the conclusion that phlogiston has the property of levity, or that it makes the compound that it is in much lighter than it would be without the phlogiston. He also showed that air was needed for combustion by putting substances in a sealed flask and trying to burn them.
Juncker published dissertations and books that developed Stahl's vitalist approach. His treatment of Stahl's work on chemical composition and reaction was "critical and coherent", making it easier to understand and reaching a greater audience. He also agreed with Stahl that the disciplines of chemistry and medicine should be treated as distinct. Juncker's Conspectus chemiae theoretico-practicae (1730) systematically explored the work of Stahl and Johann Joachim Becher, and influenced the reception of Stahl's work by eighteenth-century European thinkers including Guillaume-François Rouelle and Immanuel Kant.
As director of an orphanage and its associated medical clinic, Juncker made the practice of volunteering at the clinic (previously an option for medical students) required as part of the medical curriculum. The addition of this Collegium clinicum or practical training to the curriculum led to the expansion of both the medical program and the clinic. Under Juncker's direction, the clinic provided free medical care to thousands of poor patients each year. Juncker's work at Halle inspired the establishment of clinics at Berlin, Göttingen, Jena, and Erfurt.
Juncker wrote and published extensively. In 1721 he published Conspectus chirurgiae, an alphabetical listing describing surgical and obstetrical instruments, bandages, and other medical equipment such as a vaginal cannula for treatment of uterine prolapse. Between 1721 and 1757, 33 editions of Conspectus chirurgiae were published. It is considered one of his most important works, as is Conspectus formularum medicarum.

WORKS
- Conspectus chirurgiae : tam medicae, methodo Stahliana conscriptae, quam instrumentalis, recentissimorum auctorum ductu collectae : quae singula tabulis CIII exhibentur : adjecto indice sufficiente (33 editions 1721-1757)
- Conspectus therapiae generalis : cum notis in materiam medicam tabulis XX methodo Stahliana conscriptus (33 editions 1725-1744)
- Conspectus formularum medicarum : exhibens tabulis XVI tam methodum rationalem, quam remediorum specimina, ex praxi Stahliana potissimum desumta, et therapiae generali accomodata (24 editions 1727-2012)
- Conspectvs chemiae theoretico-practicae : in forma tabvalrvm repraesentatvs, in qvibvs physica, praesertim svbterranea, et corporvm natvralivm principia habitvs inter se, proprietates, vires et vsvs itemqve praecipva chemiae pharmacevticae et mechanicae fvndamenta e dogmatibvs Becheri et Stahlii potissimvm explicantvr, eorvndemqve et aliorvm celebrivm chemicorvm experimentis stabilivntvr (1730)
- Conspectus medicinae theoretico-practicae : tabulis CXXXVIII omnes primarios morbos methodo Stahliana tractandos, exhibens (39 editions 1718-1744)
Library resources about |
| By Johann Juncker |
|---|
pott
added details and rendered PHLOGISTON theory more approachable to the common man
Johann Heinrich Pott (1692 – 1777) was a Prussian physician and chemist. He is considered a pioneer of pyrochemistry. He examined the elements bismuth and manganese apart from attempting improvements to glass and porcelain production.
Pott, a student of one of Stahl's students, expanded the theory and attempted to make it much more understandable to a general audience. He compared phlogiston to light or fire, saying that all three were substances whose natures were widely understood but not easily defined. He thought that phlogiston should not be considered as a particle but as an essence that permeates substances, arguing that in a pound of any substance, one could not simply pick out the particles of phlogiston. Pott also observed the fact that when certain substances are burned they increase in mass instead of losing the mass of the phlogiston as it escapes; according to him, phlogiston was the basic fire principle and could not be obtained by itself. Flames were considered to be a mix of phlogiston and water, while a phlogiston-and-earthy mixture could not burn properly. Phlogiston permeates everything in the universe, it could be released as heat when combined with an acid.
Pott proposed the following properties:
1. The form of phlogiston consists of a circular movement around its axis.
2. When homogeneous it cannot be consumed or dissipated in a fire.
3. The reason it causes expansion in most bodies is unknown, but not accidental. It is proportional to the compactness of the texture of the bodies or to the intimacy of their constitution.
4. The increase of weight during calcination is evident only after a long time, and is due either to the fact that the particles of the body become more compact, decrease the volume and hence increase the density as in the case of lead, or those little heavy particles of air become lodged in the substance as in the case of powdered zinc oxide.
5. Air attracts the phlogiston of bodies.
6. When set in motion, phlogiston is the chief active principle in nature of all inanimate bodies.
7. It is the basis of colours.
8. It is the principal agent in fermentation.
Pott's formulations proposed little new theory but he supplied further details and rendered existing theory more approachable to the common man.
Pott was the son of the royal councillor Johann Andreas Pott (1662–1729) and Dorothea Sophia daughter of Andreas Machenau. He studied at the cathedral school in Halberstadt and Francke's pedagogium before studying theology at the University of Halle. He then shifted to study medicine and chemistry under Georg Ernst Stahl. In 1713, he studied assaying at Mansfield under mining master Lages. He spent two years with two of his brothers as travelling evangelists for the Community of True Inspiration but he left the sect in 1715 and returned to study chemistry at Halle, receiving a doctorate in 1716 on sulfur under Friedrich Hoffmann. He worked as a physician in Halberstadt before moving to Berlin in 1720 and became a professor of chemistry at the Collegium Medico Chirurgicum in 1724. He succeeded Caspar Neumann (1683–1737) as professor of pharmaceutical chemistry. In 1753 he attempted to get his son-in-law Ernst Gottfried Kurella into a professorship and clashed publicly with Johann Theodor Eller whose student Brandes took the position.
Pott's chemistry contributions included the use of borax and phosphorus beads in analysis. He examined graphite which he differentiated from the contemporary idea that it was lead. Pott established a porcelain factory in Freienwalde under the orders of Frederick II. He examined the composition of pyrolusite.
- Exercitationes chymicae (1738)
- Collectiones observationum et animadversionum chymicarum, 1739 and 1741
- Lithogeognosia (1746–57)
roulle
BROUGHT PHLOGISTON THEORY TO FRANCE
Guillaume François Rouelle (1703 – 1770) was a French chemist and apothecary. In 1754 he introduced the concept of a base into chemistry as a substance which reacts with an acid to form a salt. He is known as l'Aîné (the elder) to distinguish him from his younger brother, Hilaire Rouelle, who was also a chemist and known as the discoverer of urea.
Guillaume-François Rouelle brought the theory of phlogiston to France, and he was a very influential scientist and teacher so it gained quite a strong foothold very quickly. Many of his students became very influential scientists in their own right, Lavoisier included. The French viewed phlogiston as a very subtle principle that vanishes in all analysis, yet it is in all bodies. Essentially they followed straight from Stahl's theory.
Rouelle started as an apothecary. He later started a public course in his laboratory in 1738, in 1742 he was appointed experimental demonstrator of chemistry at the Jardin du Roi in Paris. he was especially influential and popular as a teacher, and taught many students among whom were Denis Diderot, Antoine-Laurent de Lavoisier, Joseph Proust and Antoine-Augustin Parmentier.
In addition to his investigation of neutral salts, he published papers on the inflammation of turpentine and other essential oils by nitric acid, and the methods of embalming practised in Ancient Egypt.
FURTHER READING
- Franckowiak, Rémi (2003). “Rouelle, un vrai-faux anti-newtonien”. Archives internationales d’histoire des sciences. 53 (150–151): 240–255.
- Franckowiak, Rémi (2002). “Les sels neutres de Guillaume-François Rouelle” (PDF). Revue d’Histoire des Sciences. 55 (4): 493–532. doi:10.3406/rhs.2002.2163.
- Lemay, Pierre; Oesper, Ralph E. (1954). “The lectures of Guillaume Francois Rouelle”. The Journal of Chemical Education. 31 (7): 338. Bibcode:1954JChEd..31..338L. doi:10.1021/ed031p338.
- Warolin, C. (1996). “Did Lavoisier benefit from the teachings of the apothecary Guillaume-François Rouelle?”. Histoire des sciences médicales. 30 (1): 30. PMID 11624829.
- Warolin, C. (1995). “Did Lavoisier benefit from the teachings of the apothecary Guillaume-François Rouelle?”. Revue d’histoire de la pharmacie. 42 (307): 361–367. doi:10.3406/pharm.1995.4257. PMID 11624913.
bergman
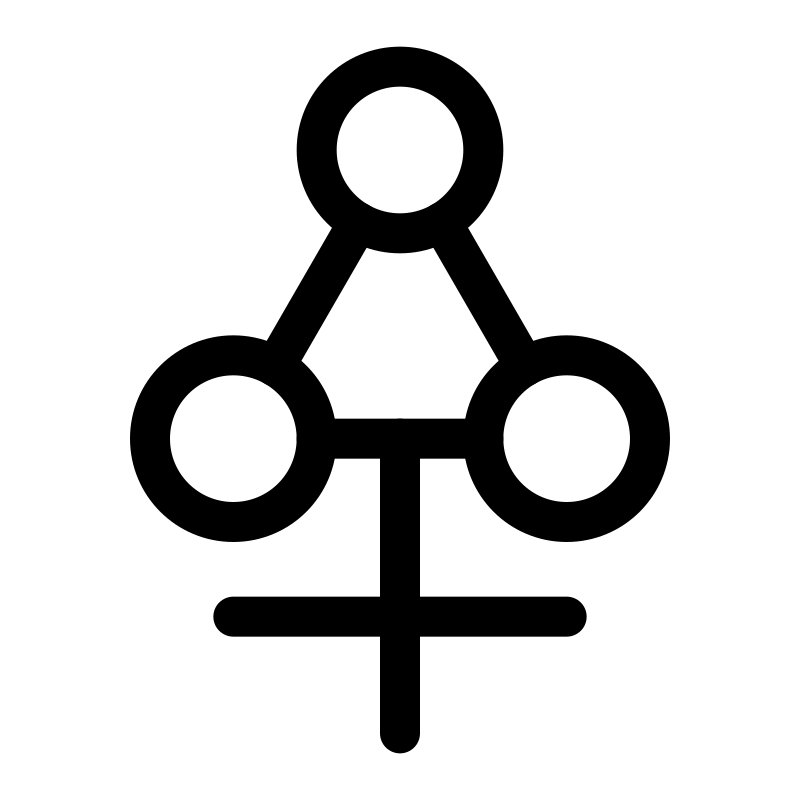
DESIGNED PHLOGISTON SYMBOLS

Torbern Olaf (Olof) Bergman (1735 –1784) was a Swedish chemist and mineralogist noted for his 1775 Dissertation on Elective Attractions, containing the largest chemical affinity tables ever published. Bergman was the first chemist to use the A, B, C, etc., system of notation for chemical species.
In 1756 he succeeded in proving that, contrary to the opinion of Linnaeus, the so-called Coccus aquaticus was really the ovum of a kind of leech.
Bergman greatly contributed to the advancement of quantitative analysis, and he developed a mineral classification scheme based on chemical characteristics and appearance. He is noted for his research on the chemistry of metals, especially bismuth and nickel.
In 1771, six years after he first discovered carbonated water and four years after Joseph Priestley first created artificially carbonated water, Bergman perfected a process to make carbonated water from chalk by the action of sulphuric acid. He is also noted for his sponsorship of Carl Wilhelm Scheele, whom some deem to be Bergman's "greatest discovery". The translation into English of his book Physical and Chemical Essays was read widely and regarded as the first systematic method of chemical analysis.
The uranium mineral torbernite and the lunar crater Bergman both bear his name.
He is said to have designed the symbol for phlogiston.
WORKS

- Physick Beskrifning Ofver Jordklotet. 1766.
- Opuscula physica et chemica (in Latin). Vol. 1. Stockholm: Magnus Swederus. 1779.
- Opuscula physica et chemica (in Latin). Vol. 2. Uppsala: John Edman. 1780.
- Opuscula physica et chemica (in Latin). Vol. 3. Leipzig: Johann Gottfried Müller. 1786.
- Opuscula physica et chemica (in Latin). Vol. 4. Leipzig: Johann Gottfried Müller. 1787.
- Opuscula physica et chemica (in Latin). Vol. 5. Leipzig: Johann Gottfried Müller. 1788.
- Bergman, Torbern (1775). A Dissertation on Elective Attractions.
- Essays, Physical and Chemical. 1779–1781.
- Historiae chemiae medium seu obscurum aevum (in Italian). Firenze: Giuseppe Tofani. 1782.
- Tekniska Museet
priestley
independent disovery of oxygen, which he called dephlogisticated air, by thermal decomposition of mercuric oxide
Joseph Priestley FRS (1733 – 1804) was an English chemist, natural philosopher, separatist theologian, grammarian, multi-subject educator, and liberal political theorist. He published over 150 works, and conducted experiments in several areas of science.

A blue plaque from the Royal Society of Chemistry commemorates Priestley at New Meeting Street, Birmingham.
Priestley is credited with his independent discovery of oxygen by the thermal decomposition of mercuric oxide, having isolated it in 1774.
During his lifetime, Priestley's considerable scientific reputation rested on his invention of carbonated water, his writings on electricity, and his discovery of several "airs" (gases), the most famous being what Priestley dubbed "dephlogisticated air" (oxygen).
Priestley's determination to defend phlogiston theory and to reject what would become the chemical revolution eventually left him isolated within the scientific community.
During the eighteenth century, as it became clear that metals gained weight after they were oxidized, phlogiston was increasingly regarded as a principle rather than a material substance.
By the end of the eighteenth century, for the few chemists who still used the term phlogiston, the concept was linked to hydrogen.
Joseph Priestley, for example, in referring to the reaction of steam on iron, while fully acknowledging that the iron gains weight after it binds with oxygen to form a calx, iron oxide, iron also loses "the basis of inflammable air (hydrogen), and this is the substance or principle, to which we give the name phlogiston".
Following Lavoisier's description of oxygen as the oxidizing principle (hence its name, from Ancient Greek: oksús, "sharp"; génos, "birth" referring to oxygen's supposed role in the formation of acids), Priestley described phlogiston as the alkaline principle.
Priestley's years in Calne were the only ones in his life dominated by scientific investigations; they were also the most scientifically fruitful. His experiments were almost entirely confined to "airs", and out of this work emerged his most important scientific texts: the six volumes of Experiments and Observations on Different Kinds of Air (1774–86). These experiments helped repudiate the last vestiges of the theory of four elements, which Priestley attempted to replace with his own variation of phlogiston theory. According to that 18th-century theory, the combustion or oxidation of a substance corresponded to the release of a material substance, phlogiston.
Priestley's work on "airs" is not easily classified. As historian of science Simon Schaffer writes, it "has been seen as a branch of physics, or chemistry, or natural philosophy, or some highly idiosyncratic version of Priestley's own invention". Furthermore, the volumes were both a scientific and a political enterprise for Priestley, in which he argues that science could destroy "undue and usurped authority" and that government has "reason to tremble even at an air pump or an electrical machine".
Volume I of Experiments and Observations on Different Kinds of Air outlined several discoveries: "nitrous air" (nitric oxide, NO); "vapor of spirit of salt", later called "acid air" or "marine acid air" (anhydrous hydrochloric acid, HCl); "alkaline air" (ammonia, NH3); "diminished" or "dephlogisticated nitrous air" (nitrous oxide, N2O); and, most famously, "dephlogisticated air" (oxygen, O2) as well as experimental findings that showed plants revitalised enclosed volumes of air, a discovery that would eventually lead to the discovery of photosynthesis.
Priestley also developed a "nitrous air test" to determine the "goodness of air". Using a pneumatic trough, he would mix nitrous air with a test sample, over water or mercury, and measure the decrease in volume—the principle of eudiometry. After a small history of the study of airs, he explained his own experiments in an open and sincere style. As an early biographer writes, "whatever he knows or thinks he tells: doubts, perplexities, blunders are set down with the most refreshing candour." Priestley also described his cheap and easy-to-assemble experimental apparatus; his colleagues therefore believed that they could easily reproduce his experiments. Faced with inconsistent experimental results, Priestley employed phlogiston theory. This led him to conclude that there were only three types of "air": "fixed", "alkaline", and "acid". Priestley dismissed the burgeoning chemistry of his day. Instead, he focused on gases and "changes in their sensible properties", as had natural philosophers before him. He isolated carbon monoxide (CO), but apparently did not realise that it was a separate "air".

More details Dumourier Dining in State at St James's, on the 15th of May, 1793 by James Gillray. Published 30 March 1793 by H. Humphrey, No. 18 Old Bond Street. “One of Gillray’s finest efforts, particularly impressive in colored impressions. Priestley bearing a mitre-crowned pie, Fox carrying the steaming head of Pitt on a platter garnished with frogs, and Sheridan holding the royal crown on a salver approach a table at which is seated the tatterdemalion figure of the French General Dumourier. Dumourier’s rumored invasion of England was never to take place but the nature of the trio’s putative allegiance and of their 'treasons in embrio' are marvelously evolved.” (British Museum # 8318).
-
Daventry Academy
-
Needham Market and Nantwich (1755–1761)
Warrington Academy (1761–1767)
- Educator and historianHistory of electricity
-
Minister of Mill Hill Chapel
-
Religious controversialist
-
Defender of Dissenters and political philosopher
-
Natural philosopher: electricity, Optics, and carbonated water
-
Materialist philosopher
-
Founder of British Unitarianism
-
Experiments and Observations on Different Kinds of Air
-
Discovery of oxygen
-
Chemical Revolution
-
Defender of English Dissenters and French revolutionaries
- Religious Activity
- Controversy
- Family Problems
- Death
WORKS
Archives
Papers of Joseph Priestley are held at the Cadbury Research Library, University of Birmingham.
Selected works
Library resources about |
| By Joseph Priestley |
|---|
- The Rudiments of English Grammar (1761)
- A Chart of Biography (1765)
- Essay on a Course of Liberal Education for Civil and Active Life (1765)
- The History and Present State of Electricity (1767)
- Essay on the First Principles of Government (1768)
- A New Chart of History (1769)
- Institutes of Natural and Revealed Religion (1772–74)
- Experiments and Observations on Different Kinds of Air (1774–77)
- Disquisitions Relating to Matter and Spirit (1777)
- The Doctrine of Philosophical Necessity Illustrated (1777)
- Letters to a Philosophical Unbeliever (1780)
- An History of the Corruptions of Christianity (1782)
- Lectures on History and General Policy (1788)
- Theological Repository (1770–73, 1784–88)
See also
- List of independent discoveries
- List of liberal theorists
- Timeline of hydrogen technologies
- Bibliography of Benjamin Franklin — Many works on Franklin make reference to Priestley

"A Word of Comfort" by William Dent (dated 22 March 1790). Priestley is preaching in front of Charles James Fox who asks "Pray, Doctor, is there such a thing as a Devil?", to which Priestley responds "No" while the devil prepares to attack Priestley from behind.

"The Friends of the People", Isaac Cruikshank (1764–1811), Scottish painter and caricaturist, London, 15 November 1792, hand-colored etching, published by S. W. Fores, No. 3 Piccadilly. In his time, Priestley’s scientific contributions were overshadowed by his Unitarian beliefs and somewhat radical views on reforming society. Here Joseph Priestley is seen seated at a table with Thomas Paine, radical supporter of the American and French revolutions, surrounded by incendiary items: guns, knives, a dish says phosphorous, a gun butt says “Royal Electric fluid.” A winged putto grins at them while squatting on the table. Thomas Paine sits upon kegs of gunpowder. Books on treason, murders, assassination, revolution, etc. surround them. At Priestley’s feet are packages of brimstone, axe, and pickax. On the walls are scenes of execution and assassinations.
lavoisier
He named oxygen, recognizED it as an element, and also recognized hydrogen as an element, opposing the phlogiston theory
Antoine-Laurent de Lavoisier (1743 – 1794), also Antoine Lavoisier after the French Revolution, was a French nobleman and chemist who was central to the 18th-century chemical revolution and who had a large influence on both the history of chemistry and the history of biology.
It is generally accepted that Lavoisier's great accomplishments in chemistry stem largely from his changing the science from a qualitative to a quantitative one.
Lavoisier is most noted for his discovery of the role oxygen plays in combustion. He named oxygen (1778), recognizing it as an element, and also recognized hydrogen as an element (1783), opposing the phlogiston theory.
He predicted the existence of silicon (1787) and discovered that, although matter may change its form or shape, its mass always remains the same.
Contributions to Chemistry
OXYGEN THEORY OF COMBUSTION
Contrary to prevailing thought at the time, Lavoisier theorized that common air, or one of its components, combines with substances when they are burned. He demonstrated this through experiment.
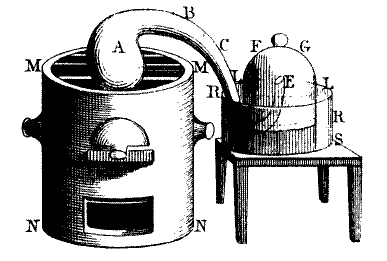
During late 1772 Lavoisier turned his attention to the phenomenon of combustion, the topic on which he was to make his most significant contribution to science. He reported the results of his first experiments on combustion in a note to the Academy on 20 October, in which he reported that when phosphorus burned, it combined with a large quantity of air to produce acid spirit of phosphorus, and that the phosphorus increased in weight on burning. In a second sealed note deposited with the Academy a few weeks later (1 November) Lavoisier extended his observations and conclusions to the burning of sulfur and went on to add that "what is observed in the combustion of sulfur and phosphorus may well take place in the case of all substances that gain in weight by combustion and calcination: and I am persuaded that the increase in weight of metallic calces is due to the same cause."[citation needed]
JOSEPH BLACK'S 'FIXED AIR'
During 1773 Lavoisier determined to review thoroughly the literature on air, particularly "fixed air," and to repeat many of the experiments of other workers in the field. He published an account of this review in 1774 in a book entitled Opuscules physiques et chimiques (Physical and Chemical Essays). In the course of this review, he made his first full study of the work of Joseph Black, the Scottish chemist who had carried out a series of classic quantitative experiments on the mild and caustic alkalies. Black had shown that the difference between a mild alkali, for example, chalk (CaCO3), and the caustic form, for example, quicklime (CaO), lay in the fact that the former contained "fixed air," not common air fixed in the chalk, but a distinct chemical species, now understood to be carbon dioxide (CO2), which was a constituent of the atmosphere. Lavoisier recognized that Black's fixed air was identical with the air evolved when metal calces were reduced with charcoal and even suggested that the air which combined with metals on calcination and increased the weight might be Black's fixed air, that is, CO2.[citation needed]
JOSEPH PRIESTLEY
In the spring of 1774, Lavoisier carried out experiments on the calcination of tin and lead in sealed vessels, the results of which conclusively confirmed that the increase in weight of metals in combustion was due to combination with air. But the question remained about whether it was in combination with common atmospheric air or with only a part of atmospheric air. In October the English chemist Joseph Priestley visited Paris, where he met Lavoisier and told him of the air which he had produced by heating the red calx of mercury with a burning glass and which had supported combustion with extreme vigor. Priestley at this time was unsure of the nature of this gas, but he felt that it was an especially pure form of common air. Lavoisier carried out his own research on this peculiar substance. The result was his memoir On the Nature of the Principle Which Combines with Metals during Their Calcination and Increases Their Weight, read to the Academy on 26 April 1775 (commonly referred to as the Easter Memoir). In the original memoir, Lavoisier showed that the mercury calx was a true metallic calx in that it could be reduced with charcoal, giving off Black's fixed air in the process. When reduced without charcoal, it gave off an air which supported respiration and combustion in an enhanced way. He concluded that this was just a pure form of common air and that it was the air itself "undivided, without alteration, without decomposition" which combined with metals on calcination.[citation needed]
After returning from Paris, Priestley took up once again his investigation of the air from mercury calx. His results now showed that this air was not just an especially pure form of common air but was "five or six times better than common air, for the purpose of respiration, inflammation, and ... every other use of common air". He called the air dephlogisticated air, as he thought it was common air deprived of its phlogiston. Since it was therefore in a state to absorb a much greater quantity of phlogiston given off by burning bodies and respiring animals, the greatly enhanced combustion of substances and the greater ease of breathing in this air were explained.[citation needed]
CHEMICAL NOMENCLATURE
CHEMICAL REVOLUTION AND OPPOSITION
Notable works
DISMANTLING PHLOGISTON THEORY
Lavoisier's chemical research between 1772 and 1778 was largely concerned with developing his own new theory of combustion. In 1783 he read to the academy his paper entitled Réflexions sur le phlogistique (Reflections on Phlogiston), a full-scale attack on the current phlogiston theory of combustion.
That year Lavoisier also began a series of experiments on the composition of water which were to prove an important capstone to his combustion theory and win many converts to it.
Many investigators had been experimenting with the combination of Henry Cavendish's inflammable air, now known as hydrogen, with "dephlogisticated air" (air in the process of combustion, now known to be oxygen) by electrically sparking mixtures of the gases. All of the researchers noted Cavendish's production of pure water by burning hydrogen in oxygen, but they interpreted the reaction in varying ways within the framework of phlogiston theory.
Lavoisier learned of Cavendish's experiment in June 1783 via Charles Blagden (before the results were published in 1784), and immediately recognized water as the oxide of a hydroelectric gas.
In cooperation with Laplace, Lavoisier synthesized water by burning jets of hydrogen and oxygen in a bell jar over mercury. The quantitative results were good enough to support the contention that water was not an element, as had been thought for over 2,000 years, but a compound of two gases, hydrogen and oxygen. The interpretation of water as a compound explained the inflammable air generated from dissolving metals in acids (hydrogen produced when water decomposes) and the reduction of calces by inflammable air (a combination of gas from calx with oxygen to form water).
Despite these experiments, Lavoisier's antiphlogistic approach remained unaccepted by many other chemists. Lavoisier labored to provide definitive proof of the composition of water, attempting to use this in support of his theory.
Working with Jean-Baptiste Meusnier, Lavoisier passed water through a red-hot iron gun barrel, allowing the oxygen to form an oxide with the iron and the hydrogen to emerge from the end of the pipe. He submitted his findings of the composition of water to the Académie des Sciences in April 1784, reporting his figures to eight decimal places.
Opposition responded to this further experimentation by stating that Lavoisier continued to draw the incorrect conclusions and that his experiment demonstrated the displacement of phlogiston from iron by the combination of water with the metal. Lavoisier developed a new apparatus which used a pneumatic trough, a set of balances, a thermometer, and a barometer, all calibrated carefully. Thirty savants were invited to witness the decomposition and synthesis of water using this apparatus, convincing many who attended of the correctness of Lavoisier's theories.
This demonstration established water as a compound of oxygen and hydrogen with great certainty for those who viewed it. The dissemination of the experiment, however, proved subpar, as it lacked the details to properly display the amount of precision taken in the measurements. The paper ended with a hasty statement that the experiment was "more than sufficient to lay hold of the certainty of the proposition" of the composition of water and stated that the methods used in the experiment would unite chemistry with the other physical sciences and advance discoveries.
THERE IS A SEPARATE NOTE COVERING THE FOLLOWING TOPICS:
BIOGRAPHY
- Early life and education
- Early scientific work
- Lavoisier as a social reformer
- Research benefitting the public good
- Sponsorship of the sciences
Ferme générale and marriage
- Adulteration of tobacco
- Royal Commission on Agriculture
- Gunpowder Commission
- During the Revolution
- Final days and execution
- Exoneration
- Oxygen theory of combustion
- Joseph Black’s “fixed air”
- Joseph Priestley
- Pioneer of stoichiometry
- Chemical nomenclature
- Chemical revolution and opposition
Notable works
- Easter memoir
- Dismantling phlogiston theory
- Elementary Treatise of Chemistry
- Physiological work
- Awards and honours

WORKS
- Opuscules physiques et chimiques (Paris: Chez Durand, Didot, Esprit, 1774). (Second edition, 1801)
- L’art de fabriquer le salin et la potasse, publié par ordre du Roi, par les régisseurs-généraux des Poudres & Salpêtres (Paris, 1779).
- Instruction sur les moyens de suppléer à la disette des fourrages, et d’augmenter la subsistence des bestiaux, Supplément à l’instruction sur les moyens de pourvoir à la disette des fourrages, publiée par ordre du Roi le 31 mai 1785 (Instruction on the means of compensating for the food shortage with fodder, and of increasing the subsistence of cattle, Supplement to the instruction on the means of providing for the food shortage with fodder, published by order of King on 31 May 1785).
- (with Guyton de Morveau, Claude-Louis Berthollet, Antoine Fourcroy) Méthode de nomenclature chimique (Paris: Chez Cuchet, 1787)
- (with Fourcroy, Morveau, Cadet, Baumé, d’Arcet, and Sage) Nomenclature chimique, ou synonymie ancienne et moderne, pour servir à l’intelligence des auteurs. (Paris: Chez Cuchet, 1789)
- Traité élémentaire de chimie, présenté dans un ordre nouveau et d’après les découvertes modernes (Paris: Chez Cuchet, 1789; Bruxelles: Cultures et Civilisations, 1965) (lit. Elementary Treatise on Chemistry, presented in a new order and alongside modern discoveries) also here
- (with Pierre-Simon Laplace) “Mémoire sur la chaleur,” Mémoires de l’Académie des sciences (1780), pp. 355–408.
- Mémoire contenant les expériences faites sur la chaleur, pendant l’hiver de 1783 à 1784, par P.S. de Laplace & A. K. Lavoisier (1792)
- Mémoires de Physique et de Chimie, de la Société d’Arcueil (1805: posthumous)
In translation
- Essays Physical and Chemical (London: for Joseph Johnson, 1776; London: Frank Cass and Company Ltd., 1970) translation by Thomas Henry of Opuscules physiques et chimiques
- The Art of Manufacturing Alkaline Salts and Potashes, Published by Order of His Most Christian Majesty, and approved by the Royal Academy of Sciences (1784) trans. by Charles Williamos[65] of L’art de fabriquer le salin et la potasse
- (with Pierre-Simon Laplace) Memoir on Heat: Read to the Royal Academy of Sciences, 28 June 1783, by Messrs. Lavoisier & De La Place of the same Academy. (New York: Neale Watson Academic Publications, 1982) trans. by Henry Guerlac of Mémoire sur la chaleur
- Essays, on the Effects Produced by Various Processes On Atmospheric Air; With A Particular View To An Investigation Of The Constitution Of Acids, trans. Thomas Henry (London: Warrington, 1783) collects these essays:
- “Experiments on the Respiration of Animals, and on the Changes effected on the Air in passing through their Lungs.” (Read to the Académie des Sciences, 3 May 1777)
- “On the Combustion of Candles in Atmospheric Air and in Dephlogistated Air.” (Communicated to the Académie des Sciences, 1777)
- “On the Combustion of Kunckel’s Phosphorus.”
- “On the Existence of Air in the Nitrous Acid, and on the Means of decomposing and recomposing that Acid.”
- “On the Solution of Mercury in Vitriolic Acid.”
- “Experiments on the Combustion of Alum with Phlogistic Substances, and on the Changes effected on Air in which the Pyrophorus was burned.”
- “On the Vitriolisation of Martial Pyrites.”
- “General Considerations on the Nature of Acids, and on the Principles of which they are composed.”
- “On the Combination of the Matter of Fire with Evaporable Fluids; and on the Formation of Elastic Aëriform Fluids.”
- “Reflections on Phlogiston”, translation by Nicholas W. Best of “Réflexions sur le phlogistique, pour servir de suite à la théorie de la combustion et de la calcination” (read to the Académie Royale des Sciences over two nights, 28 June and 13 July 1783). Published in two parts:
- Best, Nicholas W. (2015). “Lavoisier’s “Reflections on phlogiston” I: Against phlogiston theory”. Foundations of Chemistry. 17 (2): 361–378. doi:10.1007/s10698-015-9220-5. S2CID 170422925.
- Best, Nicholas W. (2016). “Lavoisier’s “Reflections on phlogiston” II: On the nature of heat”. Foundations of Chemistry. 18 (1): 3–13. doi:10.1007/s10698-015-9236-x. S2CID 94677080.
- Method of chymical nomenclature: proposed by Messrs. De Moreau, Lavoisier, Bertholet, and De Fourcroy (1788) Dictionary
- Elements of Chemistry, in a New Systematic Order, Containing All the Modern Discoveries (Edinburgh: William Creech, 1790; New York: Dover, 1965) translation by Robert Kerr of Traité élémentaire de chimie. ISBN 978-0-486-64624-4 (Dover).
- 1799 edition
- 1802 edition: volume 1, volume 2
- Some illustrations from 1793 edition
- Some more illustrations from the Science History Institute
- More illustrations (from Collected Works) from the Science History Institute
AWARDS

"The art of concluding from experience and observation consists in evaluating probabilities, in estimating if they are high or numerous enough to constitute proof. This type of calculation is more complicated and more difficult than one might think. It demands a great sagacity generally above the power of common people. The success of charlatans, sorcerors, and alchemists—and all those who abuse public credulity—is founded on errors in this type of calculation."
Antoine Lavoisier and Benjamin Franklin, Rapport des commissaires chargés par le roi de l'examen du magnétisme animal (Imprimerie royale, 1784), trans. Stephen Jay Gould, "The Chain of Reason versus the Chain of Thumbs", Bully for Brontosaurus (W.W. Norton, 1991), p. 195

Antoine Lavoisier
|
Library resources about |
| By Antoine Lavoisier |
|---|
- Archives: Fonds Antoine-Laurent Lavoisier, Le Comité Lavoisier, Académie des sciences
- Panopticon Lavoisier a virtual museum of Antoine Lavoisier
- Bibliography at Panopticon Lavoisier
- Les Œuvres de Lavoisier
- About his work
- Location of Lavoisier’s laboratory in Paris
- Radio 4 program on the discovery of oxygen by the BBC
- Who was the first to classify materials as “compounds”? – Fred Senese
- Cornell University’s Lavoisier collection
- His writings
- Works by Antoine Lavoisier at Project Gutenberg
- Works by or about Antoine Lavoisier at Internet Archive
- Les Œuvres de Lavoisier (The Complete Works of Lavoisier) edited by Pietro Corsi (Oxford University) and Patrice Bret (CNRS) (in French)
- Oeuvres de Lavoisier (Works of Lavoisier) at Gallica BnF in six volumes. (in French)
- WorldCat author page
- Title page, woodcuts, and copperplate engravings by Madame Lavoisier from a 1789 first edition of Traité élémentaire de chimie (all images freely available for download in a variety of formats from Science History Institute Digital Collections at digital.sciencehistory.org.
GIOBERT
INTRODUCED LAVOISIER'S THEORIES TO ITALY
Giovanni Antonio Giobert also known as Jean-Antoine Giobert (1761- 1834) was an Italian chemist and mineralogist who studied magnetism, galvanism, and agricultural chemistry.
He introduced Antoine Lavoisier's theories to Italy, and built a phosphorus-based eudiometer sufficiently sensitive to measure atmospheric carbon dioxide and oxygen.
He published experimental work in the debate over whether water was a simple element or chemical composition of hydrogen and oxygen. In 1792, his work on the refutation of phlogiston theory won a prize competition on the subject, put forward by the Academy of Letters and Sciences of Mantua in 1790 and 1791. His "Examen chimique de la doctrine du phlogistique et de la doctrine des pneumatistes par rapport à la nature de l 'eau", presented to the Académie royale des Sciences of Turin on 18 March 1792, is considered the most original defense of Lavoisier's theory of water composition to appear in Italy.
Giobert contributed significantly to eudiometry, the study of gas composition, by further developing Lavoisier's eudiometer. Giobert built a phosphorus-based instrument sufficiently sensitive to measure atmospheric carbon dioxide and oxygen. He used it to compare the air quality of Turin with higher altitude Vinadio. A number of other researchers developed variants on his eudiometer, including Spallanzani, Humphry Davy, John Dalton, and Joseph Louis Gay-Lussac.
In 1790, the University of Turin established the Deputazione per la Tinture, an ambitious project whose goals included the study of dye plants, the review of dyeing processes, cataloguing of dyestuffs and establishing a library, improving artisan skills, working with foreign dyers and chemists, and using new chemicals and instruments to improve the state of the art in Piedmont. An imperial decree in 1810 encouraged the improvement of scientific and industrial techniques for using woad. Giobert was active as a chemical advisor and made important contributions to the dyeing industry, studying the chemistry of natural dyes including woad, indigo, and turkey red. For example, Giobert suggested that uneven bleaching of cotton with alkaline lye was a cause of variable color-fastness when the cloth was dyed. He helped to identify differences between animal- and plant-based dyes, and developed techniques for "animalizing" fibres with nitrogen gas to improve the solidity of the dye. Such techniques became widespread throughout the European dyeing industry. In 1811 Giobert worked with Raymond Latour on the development of blue dyes which became widely used. In 1813, Giobert was appointed director of the École impériale pour la fabrication de l'indigo in Turin, which was established to study industrial processing of indigo. Giobert identified a colorless form of indigo (sometimes called indigogen or 'white' indigo) in plants, convertible to indigo-blue through oxidation. For his work on the indigo color dyeing method, Giobert was made a knight (Cavaliere).
He identified the correct composition of the mineral Gioberite, a form of magnesite (MgCO3) found in the Piedmont area. Identifying its composition was an important contribution to the industry of pottery-making.
Giobert also developed the Gioberti tincture, which involved application of hydrochloric acid and potassium ferrocyanide. The Gioberti tincture was used in the 19th century and early 20th century to restore illegible writings or faded pictures, before less harsh chemical reagents were found. Gioberti tincture was used to show the original inscriptions of palimpsests by conservators at the Vatican.
Giobert was part of a Turin-based Comitato Galvanico that supported the theories of Luigi Galvani against those of Alessandro Volta. Giobert carried out research into the conduction of electricity and the forming of precipitates along a wire in a galvanic apparatus.
The period in which Giobert was active was one of considerable political upheaval. The University of Turin was closed in both 1797 and 1799 due to political events. Giobert was a francophile and a strong republican supporter of Napoleon. In 1798 he was appointed to the provisional government of Piedmont (Il Governo Provvisorio della Nazione Piemontese), only to be imprisoned in 1799 when the Austrians briefly took power. Following his release, he became a professor at the University of Turin. In 1814, after the restoration of King Victor Emmanuel I, Giobert was one of nine professors removed from their teaching positions in Turin due to their political involvement.
Giobert died on 14 September 1834 in Millefiori near Turin. He is remembered by the town of Asti, which named a street in his honor in 1868, and established the Istituto Giobert di Asti in 1882. He was also honored by the town of Mongardino which named the Scuola Elementare Giobert di Mongardino in his honor. Scholars continue to study his life and work. A bust of Giobert was unveiled at the “Giobert: da Mongardino alla nuova chimica” conference in Mongardino on October 20, 2013.

WORKS
- Dissertazione sopra il quesito: Verificare con più accertati mezzi chimici, se l’aqua sia un corpo composto di diversi arie … oppure sia un vero elemento simplice, etc. (Dissertation on the question: Verify with more established chemical means, if the water is a body composed of different components … or it is a real simple element, etc.). Mantova (Mantua). 1794.
- Raccolta di memorie spettanti all’ agricoltura [microform] : economia di casa, commercio, arti, e manifatture (Collection of Memories Affecting Agriculture [microform]: Home Economics, Commerce, Arts, and Manufacturing). Torino (Turin): Presso Costanzo, e Fenoglio. 1791.
- “Ueber einige galvanische Versuche von Giobert. Aus einem Briefe desselben an van Mons. (On some galvanic experiments by Giobert. From a letter to van Mons.)”. Neues Allgemeines Journal der Chemie. Frölich, Berlin. 3 (2): 219. 1804.
- Traité sur le pastel et l’extraction de son Indigo. Paris: Imprimerie impériale. 1813.
- Nachricht für die Einwohner des Ober-Ems-Departements über den Anbau und die Benutzung des Waid; ein Auszug aus dem in diesem Jahre in Paris erschienenen Werke: “Traité sur le Pastel et l’extraction de son Indigo” (News for the inhabitants of the Upper Ems Department on the cultivation and use of the woad; an extract from the work published this year in Paris: “Traité sur le Pastel et l’extraction de son Indigo”.). Osnabrück: R. Koch. 1813.
- Del sovescio di segale di G.A. Giobert lettere dilucidative e comenti. Turin: Gaetano Balbino. 1819.
- Des Eaux thermales et acidules de l’Échaillon en Maurienne. Essai. Turin: Pomba et Fils. 1822.
- De l’écorce du robinier (Robinia pseudo-accacia L.), et de ses usages dans les arts et l’économie domestique (From the bark of the Robinia pseudo-accacia L.; its uses in the arts and domestic economy). Mme Huzard (Huzard née Vallat la Chapelle). 1833.
| Library resources about Giovanni Antonio Giobert |
| By Giovanni Antonio Giobert |
|---|
referencs
- https://en.wikipedia.org/wiki/Phlogiston_theory
- Wells, John C. (2008). Longman Pronunciation Dictionary (3rd ed.). Longman. ISBN 978-1-4058-8118-0.
- Mauskop, Seymour (1 November 2002). “Richard Kirwan’s Phlogiston Theory: Its Success and Fate”. Ambix. 49 (3): 185–205. doi:10.1179/amb.2002.49.3.185. ISSN 0002-6980. PMID 12833914. S2CID 170853908.
- James Bryant Conant, ed. The Overthrow of Phlogiston Theory: The Chemical Revolution of 1775–1789. Cambridge: Harvard University Press (1950), 14. OCLC 301515203.
- “Priestley, Joseph”. Spaceship-earth.de. Archived from the original on 2 March 2009. Retrieved 5 June 2009.
- Ladenburg, Dr. A (1911). Lectures on the History of Chemistry. University of Chicago Press. p. 4. Retrieved 26 August 2016.
- Bowler, Peter J (2005). Making modern science: A historical survey. Chicago: University of Chicago Press. p. 60. ISBN 9780226068602.
- Becher, Physica Subterranea p. 256 et seq.
- Brock, William Hodson (1993). The Norton history of chemistry (1st American ed.). New York: W. W. Norton. ISBN 978-0-393-03536-0.
- White, John Henry (1973). The History of Phlogiston Theory. New York: AMS Press Inc. ISBN 978-0404069308.
- Leicester, Henry M.; Klickstein, Herbert S. (1965). A Source Book in Chemistry. Cambridge, Massachusetts: Harvard University Press.
- Mason, Stephen F., (1962). A History of the Sciences (revised edition). New York: Collier Books. Ch. 26.
- Ladenburg 1911, pp. 6–7.
- “Chemistry”, Encyclopedia Britannica, 1911
- Abbri, Ferdinando (2001). “GIOBERT, Giovanni Antonio”. Dizionario Biografico degli Italiani [Biographical Dictionary of the Italians]. 55. Retrieved 15 September 2017.
- Boyle, R. A (1673). Discovery of the Perviousness of Glass to Ponderable Parts of Flame. London: Essays of Effluvium. pp. 57–85.
- For a discussion of how the term phlogiston was understood during the eighteenth century, see: James R Partington & Douglas McKie; “Historical studies on the phlogiston theory”; Annals of Science, 1937, 2, 361–404; 1938, 3, 1–58; and 337–371; 1939, 5, 113–149. Reprinted 1981 as ISBN 978-0-405-13895-9.
- Priestley, Joseph (1796). Experiments and Observations Relating to the Analysis of Atmospherical Air: Also, Farther Experiments Relating to the Generation of Air from Water. … To which are Added, Considerations on the Doctrine of Phlogiston, and the Decomposition of Water. London: J. Johnson. p. 42.
- Joseph Priestley (1794). Heads of lectures on a course of experimental philosophy. London: Joseph Johnson.
- Best, Nicholas W. (1 July 2015). “Lavoisier’s “Reflections on phlogiston” I: against phlogiston theory”. Foundations of Chemistry. 17 (2): 137–151. doi:10.1007/s10698-015-9220-5. ISSN 1572-8463. S2CID 254510272.
- Ihde, Aaron (1964). The Development of Modern Chemistry. New York: Harper & Row. p. 81.
- Rayner-Canham, Marelene; Rayner-Canham, Geoffrey (2001). Women in chemistry: their changing roles from alchemical times to the mid-twentieth century. Philadelphia: Chemical Heritage Foundation. pp. 28–31. ISBN 978-0941901277. Retrieved 2 March 2016.
- Datta, N. C. (2005). The story of chemistry. Hyderabad: Universities Press. pp. 247–250. ISBN 9788173715303. Retrieved 2 March 2016.
- Partington, J. R.; McKie, Douglas (1981). Historical Studies on the Phlogiston Theory. Arno Press. ISBN 978-0405138508.
Quotes
- Quotations related to Phlogiston theory at Wikiquote
- https://en.wikipedia.org/wiki/Phlogiston_theory
- https://en.wikipedia.org/wiki/J._J._Becher
- Bowler, Peter J (2005). Making modern science: A historical survey. Chicago: University of Chicago Press. p. 60. ISBN 9780226068602.
- Becher, Physica Subterranea p. 256 et seq.
- Brock, William Hodson (1993). The Norton history of chemistry (1st American ed.). New York: W. W. Norton. ISBN 978-0-393-03536-0.
- White, John Henry (1973). The History of Phlogiston Theory. New York: AMS Press Inc. ISBN 978-0404069308.
- Leicester, Henry M.; Klickstein, Herbert S. (1965). A Source Book in Chemistry. Cambridge, Massachusetts: Harvard University Press.
- Chisholm 1911, p. 602.
- Smith, Pamela H. (2016). The Business of Alchemy: Science and Culture in the Holy Roman Empire. Princeton: Princeton University Press. ISBN 9780691173238, p. 40/41; see also: ‘The Emperor’s Mercantile Alchemist’ in: Greenberg, Arthur (2006) – From Alchemy to Chemistry in Picture and Story. Hoboken N.J. : John Wiley & Sons. ISBN 978 0 470 08523 3. p. 231f. Chisholm writes in the 11th. ed. of the Encyclopædia Britannica that Becher “published an edition of Salzthal’s Tractatus de lapide trismegisto.”
- Chisholm 1911, pp. 602–603. …appointed physician to the elector of Bavaria; but in 1670 he was again in Vienna advising on the establishment of a silk factory and propounding schemes for a great company to trade with the Low Countries and for a canal to unite the Rhine and Danube.
- Mayr, Otto (1971). “Adam Smith and the Concept of the Feedback System: Economic Thought and Technology in 18th-Century Britain”. Technology and Culture. 12 (1): 1–22. doi:10.2307/3102276. ISSN 0040-165X. JSTOR 3102276.
- Chisholm 1911, p. 603.
- Charles W. Ingrao, The Habsburg Monarchy: 1618-1815, New York: Cambridge University Press, Second edition. ISBN 0-521-78505-7; p. 92-93. Becher was the most original and influential theorist of Austrian cameralism. He sought to balance between the need to reinstate postwar levels of population and production both in the countryside and the towns. By leaning more seriously on trade and commerce, Austrian cameralism helped to transfer attention to the troubles of the monarchy’s urban economies. Ferdinand II had already taken some corrective steps before he died by attempting to ease the debts of the Bohemian towns and to put limits on some of the land-holding nobility’s commercial rights. Even though preceding Habsburgs had held the guilds responsible for their restrictiveness, wastefulness, and the poor value of the merchandise they created, Ferdinand II ramped up the pressure by extending rights to private artisans who usually then earned the fortification of powerful local leaders such as seigneurs, military commanders, churches, and universities. An edict by Leopold I in 1689 had granted the government the right to monitor and control the number of masters and cut down on the monopoly effect of guild operations. Even previous to this, Becher, who was against all forms of monopoly, surmised that a third of the Austrian lands’ 150,000 artisans were “Schwarzarbeiter” who were not in a guild. “Immediately after the Thirty Years’ War the Bohemian towns had petitioned Ferdinand to refine its own raw materials into more finished goods for export. Becher became the leading force in attempting this conversion. By 1666 he had inspired the creation of a Commerce Commission (Kommerzkollegium) in Vienna, as well as the reestablishment of the first postwar silk plantation on the Lower Austrian estates of Hofkammer President Sinzendorf. Becher then subsequently helped create a Kunst- und Werkhaus in which foreign masters trained non-guild artisans in the production of finished goods. By 1672 he had promoted the construction of a wool factory in Linz. Four years later he established a textile workhouse for vagabonds in the Boemian town of Tabor that eventually employed 186 spinners under his own directorship.” Some of Becher’s projects met with limited success. In time Linz’s new wool factory even became one of the largest and most important in Europe. Yet most of the government initiatives ended in failure.
- Bill Bryson, A Short History of Nearly Everything, London: Black Swan, 2003 edition. ISBN 0-552-99704-8; p. 130.
- https://en.wikipedia.org/wiki/Johann_Joachim_Becherhttps
Sources
- Smith, Pamela H. (1994). The Business of Alchemy: Science and Culture in the Holy Roman Empire. Princeton: Princeton University Press.
- Ingrao, Charles W. (2005). The Habsburg Monarchy: 1618–1815. New York: Cambridge University Press.
Further reading
This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). “Becher, Johann Joachim“. Encyclopædia Britannica. Vol. 3 (11th ed.). Cambridge University Press. pp. 602–603.
- Anthony Endres, Neoclassical Microeconomic Theory: the founding Austrian version (London: Routledge Press, 1997).
- Erik Grimmer-Solem, The Rise of Historical Economics and Social Reform in Germany 1864–1894 (New York: Oxford University Press, 2003)
- Phlogiston Theory at Wikipedia
- Johann Joachim Becher at Wikipedia
- https://en.wikipedia.org/wiki/Phlogiston_theory
- https://en.wikipedia.org/wiki/Georg_Ernst_Stahl
- Stahl’s date of birth is often given erroneously as 1660. The correct date is recorded in the parish register of St. John’s church, Ansbach. See Gottlieb, B.J. (1942). “Vitalistisches Denken in Deutschland im Anschluss an Georg Ernst Stahl”. Klinische Wochenschrift. 21 (20): 445–448. doi:10.1007/bf01773817. S2CID 41987182.
- Ku-ming Chang (2008)”Stahl, Georg Ernst”, Complete Dictionary of Scientific Biography, Vol. 24, from Cengage Learning
- “Adreßbuch Deutscher Chemiker 1953/54. Gemeinsam herausgegeben von Gesellschaft Deutscher Chemiker und Verlag Chemie, Weinheim/Bergstraße. Verlag Chemie GmbH. (1953). 450 S.”. Starch – Stärke. 6 (12): 312. 1954. doi:10.1002/star.19540061211. ISSN 0038-9056.
- “Georg Ernst Stahl”. Encyclopædia Britannica, Encyclopædia Britannica Online. Encyclopædia Britannica Inc., 2013. Web. 25 May 2013
- Magill, Frank N. “Georg Ernst Stahl”, Dictionary of World Biography. 1st ed. 1999. Print.
- Francesco Paolo de Ceglia: Hoffmann and Stahl. Documents and Reflections on the Dispute. in History of Universities 22/1 (2007): 98–140.
- Smets, Alexis. The Controversy Between Leibniz and Stahl on the Theory of Chemistry Archived 2012-04-25 at the Wayback Machine, Proceedings of the 6th International Conference on History of Chemistry
- The Leibniz-Stahl Controversy (2016), transl. and edited by F. Duchesneau and J.H. Smith, Yale UP (536pp.)
- Vartanian, Aram (2006) “Stahl, Georg Ernst (1660–1734)”, Encyclopedia of Philosophy, editor Donald M. Borchert. 2nd ed. Vol. 9. Detroit: Macmillan Reference USA. 202–203. Gale Virtual Reference Library. Web. 26 May 2013.
- Chang, K (2004). “Motus Tonicus: Georg Ernst Stahl’s Formulation of Tonic Motion and Early Modern Medical Thought”. Bulletin of the History of Medicine. 78 (4): 767–803. doi:10.1353/bhm.2004.0161. PMID 15591695. S2CID 12488842. Retrieved 20 April 2016.
- Hudson, John (1992). The History of Chemistry. Hong Kong: The Macmillan Press. p. 47. ISBN 0-412-03641-X.
- Hopper, Christopher P.; Zambrana, Paige N.; Goebel, Ulrich; Wollborn, Jakob (June 2021). “A brief history of carbon monoxide and its therapeutic origins”. Nitric Oxide. 111–112: 45–63. doi:10.1016/j.niox.2021.04.001.
- Hélène Metzger (1926) “La philosophie de la matière chez Stahl et ses disciples” Isis >8: 427–464.
- Hélène Metzger (1930) Newton, Stahl, Boerhaave et la Doctrine Chemique
- Lawrence M. Principe (2007) Chymists and Chymistry
- White, John Henry (1973). The History of Phlogiston Theory. New York: AMS Press Inc. ISBN 978-0404069308.
- Leicester, Henry M.; Klickstein, Herbert S. (1965). A Source Book in Chemistry. Cambridge, Massachusetts: Harvard University Press.
- Mason, Stephen F., (1962). A History of the Sciences (revised edition). New York: Collier Books. Ch. 26.Ladenburg 1911, pp. 6–7.
- Phlogiston at Wikipedia
- Georg Ernst Stahl at Wikipedia
External links
- https://en.wikipedia.org/wiki/Phlogiston_theory
- https://en.wikipedia.org/wiki/Antoine-Laurent_de_Lavoisier
- “Lavoisier, Antoine Laurent”. Lexico UK English Dictionary. Oxford University Press. Archived from the original on 23 April 2021.
- “Lavoisier”. Collins English Dictionary. HarperCollins. Retrieved 30 July 2019.
- “Lavoisier”. Merriam-Webster.com Dictionary. Retrieved 30 July 2019.
- (in French) Lavoisier, le parcours d’un scientifique révolutionnaire CNRS (Centre National de la Recherche Scientifique)
- Schwinger, Julian (1986). Einstein’s Legacy. New York: Scientific American Library. p. 93. ISBN 978-0-7167-5011-6.
- In his table of the elements, Lavoisier listed five “salifiable earths” (i.e., ores that could be made to react with acids to produce salts (salis = salt, in Latin)): chaux (calcium oxide), magnésie (magnesia, magnesium oxide), baryte (barium sulfate), alumine (alumina, aluminium oxide), and silice (silica, silicon dioxide). About these “elements”, Lavoisier speculates: “We are probably only acquainted as yet with a part of the metallic substances existing in nature, as all those which have a stronger affinity to oxygen than carbon possesses, are incapable, hitherto, of being reduced to a metallic state, and consequently, being only presented to our observation under the form of oxyds, are confounded with earths. It is extremely probable that barytes, which we have just now arranged with earths, is in this situation; for in many experiments it exhibits properties nearly approaching to those of metallic bodies. It is even possible that all the substances we call earths may be only metallic oxyds, irreducible by any hitherto known process.” – from p. 218 of: Lavoisier with Robert Kerr, trans., Elements of Chemistry, …, 4th ed. (Edinburgh, Scotland: William Creech, 1799). (The original passage appears in: Lavoisier, Traité Élémentaire de Chimie, … (Paris, France: Cuchet, 1789), vol. 1, p. 174.)
- Schama, Simon (1989). Citizens: A Chronicle of the French Revolution. Alfred A Knopf. p. 73.
- Herbermann, Charles, ed. (1913). “Antoine-Laurent Lavoisier” . Catholic Encyclopedia. New York: Robert Appleton Company.
- Chisholm, Hugh, ed. (1911). “Lavoisier, Antoine Laurent” . Encyclopædia Britannica. Vol. 16 (11th ed.). Cambridge University Press. p. 295.
- Yount, Lisa (2008). Antoine Lavoisier : founder of modern chemistry. Berkeley Heights, NJ: Enslow Publishers. p. 115. ISBN 978-0-7660-3011-4. Retrieved 25 July 2016.
- Duveen, Dennis I. (1965). Supplement to a bibliography of the works of Antoine Laurent Lavoisier, 1743–1794. London: Dawsons.
- McKie, Douglas (1935). Bibliographic Details Antoine Lavoisier, the father of modern chemistry, by Douglas McKie … With an introduction by F.G. Donnan. London: V. Gollancz ltd.
- Bibliographic Details Lavoisier in perspective / edited by Marco Beretta. Munich: Deutsches Museum. 2005.
- Bell, Madison Smart (2005). Lavoisier in the year one. New York: W.W. Norton.
- McKie, Douglas (1952). Antoine Lavoisier: scientist, economist, social reformer. New York: Schuman.
- Citizens, Simon Schama. Penguin 1989 p. 236
- Eagle, Cassandra T.; Jennifer Sloan (1998). “Marie Anne Paulze Lavoisier: The Mother of Modern Chemistry”. The Chemical Educator. 3 (5): 1–18. CiteSeerX 10.1.1.472.7513. doi:10.1007/s00897980249a. S2CID 97557390.
- Donovan, Arthur (1996). Antoine Lavoisier: Science, Administration, and Revolution. Cambridge: Cambridge University Press. p. 273. ISBN 978-0-521-56672-8.
- Jean-Pierre Poirier (1998). Lavoisier: Chemist, Biologist, Economist. University of Pennsylvania Press. pp. 24–26. ISBN 978-0-8122-1649-3.
- W.R. Aykroyd (12 May 2014). Three Philosophers: Lavoisier, Priestley and Cavendish. Elsevier Science. pp. 168–170. ISBN 978-1-4831-9445-5.
- Arthur Donovan (11 April 1996). Antoine Lavoisier: Science, Administration and Revolution. Cambridge University Press. pp. 123–125. ISBN 978-0-521-56672-8.
- Citizens, Simon Schama, Penguin 1989 p. 313
- Dutton, William S. (1942), Du Pont: One Hundred and Forty Years, Charles Scribner’s Sons, LCCN 42011897.
- Chronicle of the French Revolution, Jacques Legrand, Longman 1989, p. 216 ISBN 0-582-05194-0
- Companion to the French Revolution, John Paxton, Facts on File Publications 1988, p. 120
- A Cultural History of the French Revolution, Emmet Kennedy, Yale University Press 1989, p. 193
- Chronicle of the French Revolution, Jacques Legrand, Longman 1989, p. 204 ISBN 0-582-05194-0
- Chronicle of the French Revolution, Jacques Legrand, Longman 1989, p. 356 ISBN 0-582-05194-0
- Chronicle of the French Revolution, Jacques Legrand, Longman 1989 ISBN 0-582-05194-0[page needed]
- O’Connor, J.J.; Robertson, E.F. (26 September 2006). “Joseph-Louis Lagrange”. Archived from the original on 2 May 2006. Retrieved 20 April 2006.
In September 1793 a law was passed ordering the arrest of all foreigners born in enemy countries and all their property to be confiscated. Lavoisier intervened on behalf of Lagrange, who certainly fell under the terms of the law. On 8 May 1794, after a trial that lasted less than a day, a revolutionary tribunal condemned Lavoisier and 27 others to death. Lagrange said on the death of Lavoisier, who was guillotined on the afternoon of the day of his trial
- Chronicle of the French Revolution, Longman 1989 p. 202 ISBN 0-582-05194-0
- “Today in History: 1794: Antoine Lavoisier, the father of modern chemistry, is executed on the guillotine during France’s Reign of Terror”. Archived from the original on 15 June 2013.
- Commenting on this quotation, Denis Duveen, an English expert on Lavoiser and a collector of his works, wrote that “it is pretty certain that it was never uttered”. For Duveen’s evidence, see the following: Duveen, Denis I. (February 1954). “Antoine Laurent Lavoisier and the French Revolution”. Journal of Chemical Education. 31 (2): 60–65. Bibcode:1954JChEd..31…60D. doi:10.1021/ed031p60
- Delambre, Jean-Baptiste (1867). Œuvres de Lagrange (in French). Gauthier-Villars. 15–57 – via Wikisource.
- Guerlac, Henry (1973). Antoine-Laurent Lavoisier – Chemist and Revolutionary. New York: Charles Scribner’s Sons. p. 130.
- (In French) M.-A. Paulze, épouse et collaboratrice de Lavoisier, Vesalius, VI, 2, 105–113, 2000, p. 110. (PDF)
- Guerlac, Henry (2019). “Lavoisier—the Crucial Year: The Background and Origin of His First Experiments on Combustion in 1772”. Cornell University Press. doi:10.7298/GX84-1A09.
- Lavoisier, Antoine (1777) “Mémoire sur la combustion en général” Archived 17 June 2013 at the Wayback Machine (“On Combustion in General”). Mémoires de l’Académie des sciences. English translation
- Petrucci R.H., Harwood W.S. and Herring F.G., General Chemistry (8th ed. Prentice-Hall 2002), p. 34
- “An Historical Note on the Conservation of Mass”.
- Duveen, Denis; Klickstein, Herbert (September 1954). “The Introduction of Lavoisier’s Chemical Nomenclature into America”. The History of Science Society. 45 (3).
- “Traité élémentaire de chimie : Présenté dans un ordre nouveau et d’après les découvertes modernes ; avec figures”. A Paris, Chez Cuchet, libraire, rue et Hôtel Serpente. 1789.
- Holleman, A. F.; Wiberg, Egon; Wiberg, Nils (2001). Inorganic Chemistry. Academic Press. p. 17. ISBN 978-0-12-352651-9.
- Golinski, Jan (1994). “Precision instruments and the demonstrative order of proof in Lavoisier’s chemistry” (PDF). Osiris. 9: 30–47. doi:10.1086/368728. JSTOR 301997. S2CID 95978870.
- Kirwan, Essay on Phlogiston, viii, xi.
- Lavoisier, Antoine (1778) “Considérations générales sur la nature des acides” Archived 17 June 2013 at the Wayback Machine (“General Considerations on the Nature of Acids”). Mémoires de l’Académie des sciences. lavoisier.cnrs.fr
- Gillispie, Charles Coulston (1960). The Edge of Objectivity: An Essay in the History of Scientific Ideas. Princeton University Press. p. 228. ISBN 0-691-02350-6.
- Lavoisier and Meusnier, “Développement” (cit. n. 27), pp. 205–209; cf. Holmes, Lavoisier (cn. 8), p. 237.
- See the “Advertisement,” p. vi of Kerr’s translation, and pp. xxvi–xxvii, xxviii of Douglas McKie’s introduction to the Dover edition.
- Is a Calorie a Calorie? American Journal of Clinical Nutrition, Vol. 79, No. 5, 899S–906S, May 2004
- Levere, Trevor (2001). Transforming Matter. Maryland: The Johns Hopkins University Press. pp. 72–73. ISBN 978-0-8018-6610-4.
- Gillespie, Charles C. (1996), Foreword to Lavoisier by Jean-Pierre Poirier, University of Pennsylvania Press, English Edition.
- Beretta, Marco (8 June 2022). The Arsenal of Eighteenth-Century Chemistry:The Laboratories of Antoine Laurent Lavoisier (1743-1794). Koninklijke Brill. p. 107. ISBN 9789004511217. Archived from the original on 24 December 2023. Retrieved 24 December 2023.
- “Place name detail: Mount Lavoisier”. New Zealand Gazetteer. New Zealand Geographic Board. Retrieved 21 August 2022.
- “APS Member History”. search.amphilsoc.org. Retrieved 28 May 2021.
- “Antoine-Laurent Lavoisier: The Chemical Revolution”. National Historic Chemical Landmarks. American Chemical Society. Archived from the original on 23 February 2013. Retrieved 25 March 2013.
- Guyton de Morveau, Louis Bernard; Lavoisier, Antoine Laurent; Berthollet, Claude-Louis; Fourcroy, Antoine-François de (1787). Méthode de Nomenclature Chimique. Paris, France: Chez Cuchet (Sous le Privilége de l’Académie des Sciences).
- “2015 Awardees”. American Chemical Society, Division of the History of Chemistry. University of Illinois at Urbana-Champaign School of Chemical Sciences. 2015. Retrieved 1 July 2016.
- “Citation for Chemical Breakthrough Award” (PDF). American Chemical Society, Division of the History of Chemistry. University of Illinois at Urbana-Champaign School of Chemical Sciences. 2015. Archived (PDF) from the original on 9 October 2022. Retrieved 1 July 2016.
- “Société Chimique de France”. www.societechimiquedefrance.fr. Archived from the original on 29 March 2019. Retrieved 28 March 2019.
- “International Society for Biological Calorimetry (ISBC) – About ISBC_”. biocalorimetry.ucoz.org. Retrieved 28 March 2019.
- workflow-process-service. “The Lavoisier Medal honors exceptional scientists and engineers | DuPont USA”. www.dupont.com. Retrieved 28 March 2019.
- “Le Prix Franklin–Lavoiser2018 a été décerné au Comité Lavoisier”. La Gazette du Laboratoire. 20 June 2018. Retrieved 15 January 2019.
- “Franklin-Lavoisier Prize”. Science History Institute. Archived from the original on 26 March 2020. Retrieved 26 March 2020.
- See Denis I. Duveen and Herbert S. Klickstein, “The “American” Edition of Lavoisier’s L’art de fabriquer le salin et la potasse,” The William and Mary Quarterly, Third Series 13:4 (October 1956), 493–498.
Further reading
- Herbermann, Charles, ed. (1913). “Antoine-Laurent Lavoisier” . Catholic Encyclopedia. New York: Robert Appleton Company.
- Bailly, J.-S., “Secret Report on Mesmerism or Animal Magnetism”, International Journal of Clinical and Experimental Hypnosis, Vol. 50, No. 4, (October 2002), pp. 364–368. doi:10.1080/00207140208410110
- Berthelot, M. (1890). La révolution chimique: Lavoisier. Paris: Alcan.
- Catalogue of Printed Works by and Memorabilia of Antoine Laurent Lavoisier, 1743–1794… Exhibited at the Grolier Club (New York, 1952).
- Daumas, M. (1955). Lavoisier, théoricien et expérimentateur. Paris: Presses Universitaires de France.
- Donovan, Arthur (1993). Antoine Lavoisier: Science, Administration, and Revolution. Cambridge, England: Cambridge University Press.
- Duveen, D.I. and H.S. Klickstein, A Bibliography of the Works of Antoine Laurent Lavoisier, 1743–1794 (London, 1954)
- Franklin, B., Majault, M.J., Le Roy, J.B., Sallin, C.L., Bailly, J.-S., d’Arcet, J., de Bory, G., Guillotin, J.-I. & Lavoisier, A., “Report of The Commissioners charged by the King with the Examination of Animal Magnetism”, International Journal of Clinical and Experimental Hypnosis, Vol.50, No.4, (October 2002), pp. 332–363. doi:10.1080/00207140208410109
- Grey, Vivian (1982). The Chemist Who Lost His Head: The Story of Antoine Lavoisier. Coward, McCann & Geoghegan, Inc. ISBN 9780698205598.
- Guerlac, Henry (1961). Lavoisier – The Crucial Year. Ithaca, New York: Cornell University Press.
- Holmes, Frederic Lawrence (1985). Lavoisier and the Chemistry of Life. Madison, Wisconsin: University of Wisconsin Press.
- Holmes, Frederic Lawrence (1998). Antoine Lavoisier – The Next Crucial Year, or the Sources of his Quantitative Method in Chemistry. Princeton University Press.
- Jackson, Joe (2005). A World on Fire: A Heretic, An Aristocrat And The Race to Discover Oxygen. Viking.
- Johnson, Horton A. (2008). “Revolutionary Instruments, Lavoisier’s Tools as Objets d’Art”. Chemical Heritage Magazine. 26 (1): 30–35.
- Kelly, Jack (2004). Gunpowder: Alchemy, Bombards, & Pyrotechnics. Basic Books. ISBN 978-0-465-03718-6.
- McKie, Douglas (1935). Antoine Lavoisier: The Father of Modern Chemistry. Philadelphia: J. B. Lippincott & Co.
- McKie, Douglas (1952). Antoine Lavoisier: Scientist, Economist, Social Reformer. New York: Henry Schuman.
- Poirier, Jean-Pierre (1996). Lavoisier (English ed.). University of Pennsylvania Press.
- Scerri, Eric (2007). The Periodic Table: Its Story and Its Significance. Oxford University Press. ISBN 978-0-19-530573-9.
- Smartt Bell, Madison (2005). Lavoisier in the Year One: The Birth of a New Science in an Age of Revolution. Atlas Books, W.W. Norton.
- https://en.wikipedia.org/wiki/Phlogiston_theory
- https://en.wikipedia.org/wiki/Phlogiston_theory
- https://en.wikipedia.org/wiki/Phlogiston_theory
- https://en.wikipedia.org/wiki/Joseph_Priestley
- “List of Fellows of the Royal Society 1660 – 2007, K – Z”. royalsociety.org. The Royal Society. Archived from the original on 12 December 2007. Retrieved 1 August 2019.
- “Copley archive winners 1799–1731”. royalsociety.org. The Royal Society. Archived from the original on 11 January 2008. Retrieved 1 August 2019.
- “Priestley” Archived 30 October 2014 at the Wayback Machine: Collins English Dictionary – Complete & Unabridged 2012 Digital Edition.
- Gray & Harrison: Oxford Dictionary of National Biography, pp. 351-352
- Isaacson, 2004, pp. 140–141, 289
- Schofield, 1997, p. 142
- H. I. Schlesinger (1950). General Chemistry (4th ed.). p. 134.
- Although Swedish chemist Carl Wilhelm Scheele also has strong claims to the discovery, Priestley published his findings first. Scheele discovered it by heating potassium nitrate, mercuric oxide, and many other substances in about 1772.
- “Joseph Priestley, Discoverer of Oxygen National Historic Chemical Landmark”. American Chemical Society. Retrieved 21 May 2021.
- Tapper, 10.
- Tapper, 314.
- Van Doren, p. 420
- Schofield, 1997, p. 274
- Schofield (1997), 2.
- Schofield (1997), 2–12; Uglow, 72; Jackson, 19–25; Gibbs, 1–4; Thorpe, 1–11; Holt, 1–6.
- Schofield (1997), 1, 7–8; Jackson, 25–30; Gibbs, 4; Priestley, Autobiography, 71–73, 123.
- Schofield (1997), 14, 28–29; Uglow, 72; Gibbs, 5; Thorpe, 11–12; Holt, 7–9.
- Schofield (1997), 28–29; Jackson, 30; Gibbs, 5.
- McEvoy (1983), 48–49.
- Qtd. in Jackson, 33. See Schofield (1997), 40–57; Uglow, 73–74; Jackson, 30–34; Gibbs, 5–10; Thorpe, 17–22; Tapper, 314; Holt, 11–14; Garrett, 54.
- Schofield (1997), 62–69.
- Schofield (1997), 62–69; Jackson, 44–47; Gibbs, 10–11; Thorpe, 22–29; Holt, 15–19.
- Priestley, Joseph. The Rudiments of English Grammar; adapted to the use of schools. With observations on style. London: Printed for R. Griffiths, 1761.
- Qtd. in Schofield (1997), 79.
- Schofield (1997), 77–79, 83–85; Uglow, 72; Jackson 49–52; Gibbs, 13–16; Thorpe, 30–32; Holt, 19–23.
- McLachlan, Iconography, 24–26.
- Schofield, Robert E. (2009). Enlightened joseph priestley : a study of his life and work from 1773 to 1804. University Park: Penn State Univ Press. ISBN 978-0-271-03625-0. Retrieved 26 June 2018.
- Meyer, Michal (2018). “Old Friends”. Distillations. 4 (1): 6–9. Retrieved 26 June 2018.
- Bowden, Mary Ellen; Rosner, Lisa, eds. (2005). Joseph Priestley, radical thinker : a catalogue to accompany the exhibit at the Chemical Heritage Foundation commemorating the 200th anniversary of the death of Joseph Priestley, 23 August 2004 to 29 July 2005. Philadelphia, Penns.: Chemical Heritage Foundation. p. 26. ISBN 978-0941901383. Archived from the original on 2 June 2016. Retrieved 11 September 2014.
- Priestley, Autobiography, 87.
- See Thorpe, 33–44 for a description of life at Warrington; Schofield (1997), 89–90, 93–94; Jackson, 54–58; Uglow, 73–75; Thorpe, 47–50; Holt, 27–28.
- Sheps, 135, 149; Holt, 29–30.
- Qtd. in Sheps, 146.
- Priestley, Joseph. Essay on a Course of Liberal Education for Civil and Active Life. London: Printed for C. Henderson under the Royal Exchange; T. Becket and De Hondt in the Strand; and by J. Johnson and Davenport, in Pater-Noster-Row, 1765.
- Thorpe, 52–54; Schofield (1997), 124–25; Watts, 89, 95–97; Sheps, 136.
- Schofield (1997), 121; see also Watts, 92.
- Schofield (2004), 254–59; McLachlan (1987–90), 255–58; Sheps, 138, 141; Kramnick, 12; Holt, 29–33.
- Priestley, Joseph. A Chart of Biography. London: J. Johnson, St. Paul’s Church Yard, 1765 and Joseph Priestley, A Description of a Chart of Biography. Warrington: Printed by William Eyres, 1765 and Joseph Priestley, A New Chart of History. London: Engraved and published for J. Johnson, 1769; A Description of a New Chart of History. London: Printed for J. Johnson, 1770.
- Rosenberg, 57–65 and ff.
- Gibbs, 37; Schofield (1997), 118–19.
- J. Priestley. A Course of Lectures on Oratory and Criticism. London, 1777. Ed. V. M. Bevilacqua & R. Murphy. Carbondale: Southern Illinois University Press, 1965.
- Schofield (1997), 136–37; Jackson, 57–61.
- Isaacson, 2004, pp. 140–141, 182
- Van Doren, pp. 164–165
- Schofield (1997), 141–42, 152; Jackson, 64; Uglow 75–77; Thorpe, 61–65.
- Schofield (1997), 143–44; Jackson, 65–66; see Schofield (1997), 152 and 231–32 for an analysis of the different editions.
- Priestley, Joseph. The History and Present State of Electricity, with original experiments. London: Printed for J. Dodsley, J. Johnson and T. Cadell, 1767.
- Schofield (1997), 144–56.
- Schofield (1997), 156–57; Gibbs 28–31; see also Thorpe, 64.
- Other early investigators who suspected that the electrical force diminished with distance as the gravitational force did (i.e., as the inverse square of the distance) included Daniel Bernoulli (see: Abel Socin (1760) Acta Helvetia, vol. 4, pp. 224–25.) and Alessandro Volta, both of whom measured the force between plates of a capacitor, and Aepinus. See: J.L. Heilbron, Electricity in the 17th and 18th Centuries: A Study of Early Modern Physics (Los Angeles, California: University of California Press, 1979), pp. 460–62, 464 Archived 14 May 2016 at the Wayback Machine (including footnote 44).
- Joseph Priestley, The History and Present State of Electricity, with Original Experiments (London, England: 1767), p. 732 Archived 28 May 2016 at the Wayback Machine:
May we not infer from this experiment, that the attraction of electricity is subject to the same laws with that of gravitation, and is therefore according to the squares of the distances; since it is easily demonstrated, that were the earth in the form of a shell, a body in the inside of it would not be attracted to one side more than another?
- Coulomb (1785) “Premier mémoire sur l’électricité et le magnétisme,” Histoire de l’Académie Royale des Sciences, pp. 569–577
- Priestley, Joseph. A Familiar Introduction to the Study of Electricity. London: Printed for J. Dodsley; T. Cadell; and J. Johnson, 1768.
- Schofield (1997), 228–30.
- Schofield (1997), 162–64.
- Priestley, Autobiography, 98; see also Schofield (1997), 163.
- Schofield (1997), 162, note 7.
- Schofield, (1997), 158, 164; Gibbs, 37; Uglow, 170.
- Schofield (1997), 165–69; Holt, 42–43.
- Schofield (1997), 170–71; Gibbs, 37; Watts, 93–94; Holt, 44.
- Priestley. Institutes of Natural and Revealed Religion. London: Printed for J. Johnson, Vol. I, 1772, Vol. II, 1773, Vol. III, 1774.
- Miller, xvi; Schofield (1997), 172.
- Schofield (1997), 174; Uglow, 169; Tapper, 315; Holt, 44.
- Qtd. in Jackson, 102.
- McLachlan (1987–90), 261; Gibbs, 38; Jackson, 102; Uglow, 169.
- Schofield (1997), 181.
- See Schofield (1997), 181–88 for analysis of these two controversies.
- See Schofield (1997), 193–201 for an analysis of the journal; Uglow, 169; Holt, 53–55.
- See Schofield (2004), 202–7 for an analysis of Priestley’s contributions.
- Schofield (1997), 207.
- Schofield (1997), 202–05; Holt, 56–64.
- Priestley, Joseph. Essay on the First Principles of Government; and on the nature of political, civil, and religious liberty. London: Printed for J. Dodsley; T. Cadell; and J. Johnson, 1768.
- Gibbs, 39–43; Uglow, 169; Garrett, 17; Tapper, 315; Holt, 34–37; Philip (1985); Miller, xiv.
- Priestley, Joseph. Remarks on some paragraphs in the fourth volume of Dr. Blackstone’s Commentaries on the laws of England, relating to the Dissenters. London: Printed for J. Johnson and J. Payne, 1769.
- Schofield (1997), 214–16; Gibbs, 43; Holt, 48–49.
- Qtd. in Kramnick, 8.
- Kramnick, 1981, p. 10
- Schofield (1997), 227, 232–38; see also Gibbs, 47; Kramnick, 9–10.
- Priestley, Joseph. Proposals for printing by subscription, The history and present state of discoveries relating to vision, light, and colours. Leeds: n.p., 1771.
- Moura, Breno (2018). “Newtonian Optics and the Historiography of Light in the 18th Century: A critical Analysis of Joseph Priestley’s The History of Optics”. Transversal: International Journal for the Historiography of Science (5). doi:10.24117/2526-2270.2018.i5.12. ISSN 2526-2270. S2CID 239593348.
- Schofield (1997), 240–49; Gibbs, 50–55; Uglow, 134.
- Priestley, Joseph. Directions for impregnating water with fixed air; in order to communicate to it the peculiar spirit and virtues of Pyrmont water, and other mineral waters of a similar nature. London: Printed for J. Johnson, 1772.
- Schofield (1997), 256–57; Gibbs, 57–59; Thorpe, 76–79; Uglow, 134–36; 232–34.
- Schils, René (2011). How James Watt Invented the Copier: Forgotten Inventions of Our Great Scientists. Springer Science & Business Media. p. 36.
- LaMoreaux, Philip E. (2012). Springs and Bottled Waters of the World: Ancient History, Source, Occurrence, Quality and Use. Springer Science & Business Media. p. 135.
- Schofield (1997), 251–55; see Holt, 64; Gibbs, 55–56; and Thorpe, 80–81, for the traditional account of this story.
- Schofield (1997), 270–71; Jackson, 120–22; Gibbs, 84–86: Uglow, 239–40; Holt, 64–65.
- McLachlan, Iconography, 19–20.
- Qtd. in Gibbs, 91.
- Schofield (2004), 4–11; 406; Gibbs, 91–94; Jackson, 122, 124, 143–52, 158–62; Thorpe, 80–85; Watts, 96; Holt, 70–94 (includes large quotations from Priestley’s letters sent from Europe to Shelburne’s sons).
- McEvoy and McGuire, 326–27; Tapper, 316.
- Schofield (2004), 72.
- Schofield (2004), 59–76; Gibbs, 99–100; Holt, 112–24; McEvoy and McGuire, 333–34.
- Tapper, 320; Priestley, Autobiography, 111; Schofield (2004), 37–42; Holt, 93–94; 139–42.
- Schofield (2004), 77–91; Garrett, 55; Tapper, 319; Sheps, 138; McEvoy (1983), 50; McEvoy and McGuire, 338–40.
- Sheps, 138.
- McEvoy and McGuire, 341–45.
- Leibniz, Gottfried Wilhelm. Confessio Philosophi: Papers Concerning the Problem of Evil, 1671–1678. Trans. Robert C. Sleigh, Jr. New Haven: Yale University Press (2004), xxxviii, 109. ISBN 978-0-300-08958-5. The original Latin text and an English translation of Leibniz’s A Philosopher’s Creed can be found on the Latin and English Wikisources, respectively.
- Stewart, Matthew. The Courtier and the Heretic: Leibniz, Spinoza, and the Fate of God in the Modern World. New York: W. W. Norton (2006), 171. ISBN 0-393-05898-0.
- McEvoy and McGuire, 341.
- Adams, Robert Merrihew. Leibniz: Determinist, Theist, Idealist. New York: Oxford University Press (1998), 10–13, 1–20, 41–44. ISBN 0-19-508460-8.
- Rutherford, 213–18.
- Rutherford, 46.
- Schofield (2004), 78–79.
- Rutherford, 12–15, 22–45, 49–54.
- Priestley, Joseph. Letter to a Layman, on the Subject of the Rev. Mr. Lindsey’s Proposal for a Reformed English Church. London: Printed for J. Wilkie, 1774.
- Schofield (2004), 26–28; Jackson, 124; Gibbs, 88–89; Holt, 56–64.
- Schofield (2004), 225, 236–38.
- Priestley, Joseph. Experiments and Observations on Different Kinds of Air. 3 vols. London W. Bowyer and J. Nichols, 1774–77. There are several different editions of these volumes, each important.
- See Gibbs 67–83 for a description of all of Priestley’s experiments during this time; Thorpe, 170ff.
- Thorpe, 167–68; Schofield (2004), 98–101.
- Schaffer, 152.
- Qtd. in Kramnick, 11–12; see also Schofield (2004), 121–24.
- Fruton, 20, 29
- Schofield (2004), 98; Thorpe, 171.
- Schofield (1997), 259–69; Jackson, 110–14; Thorpe, 76–77, 178–79; Uglow, 229–39.
- Schofield (2004), 93–105; Uglow, 240–41; see Gibbs 105–16 for a description of these experiments.
- Priestley, Joseph. “An Account of Further Discoveries in Air”. Philosophical Transactions 65 (1775): 384–94.
- Qtd. in Schofield (2004), 107.
- Wagner, P. (2012). Hypoxia. Springer. p. 10. ISBN 978-1-4419-8997-0. Retrieved 2 January 2024.
- Schofield (2004), 105–19; see also Jackson, 126–27, 163–64, 166–74; Gibbs, 118–23; Uglow, 229–31, 241; Holt, 93.
- Kuhn, 53–55.
- Schofield (2004), 129–30; Gibbs, 124–25.
- Schofield (2004), 141–43; see also Jackson, 198–99; Holt, 81–82.
- Nikkah, Roya (16 December 2012). “The Duchess discovers blue blood in her own family”. UK Sunday Telegraph. p. 9. Archived from the original on 29 October 2014. Retrieved 8 July 2014.
- Schofield (2004), 147–50, 196–99, 242–46. Gibbs, 134–40, 169; Uglow, 310–20, 407; Jackson, 227–28; Holt, 132–33.
- “Book of Members, 1780–2010: Chapter P” (PDF). American Academy of Arts and Sciences. Archived (PDF) from the original on 15 May 2011. Retrieved 28 July 2014.
- Schofield (2004), 151–52; for an analysis of Priestley’s contributions to each man’s work, see Schofield’s chapter “Science and the Lunar Society”; see also Jackson, 200–01; Gibbs, 141–47; Thorpe, 93–102; Holt, 127–32; Uglow, 349–50; for a history of the Lunar Society, see Uglow.
- Qtd. in Schofield (2004), 167
- Schofield (2004), 168; see also Jackson 203–08; Gibbs, 154–61; Uglow, 358–61.
- Memoirs of the Royal Academy of Sciences of Paris année 1777 (1780): 592–600. The next, most notable installment was “Réflexions sur le phlogistique, pour servir de suite à la théorie de la combustion et de la calcination publiée en 1777” Memoirs of the Royal Academy of Sciences of Paris année 1783 (1786): 505–538 (translated by Nicholas W. Best as “Lavoisier’s ‘Reflections on Phlogiston’ I: Against Phlogiston Theory”, Foundations of Chemistry 17 (2015): 137–151).
- Thorpe, 210; see also Schofield (2004), 169–94; Jackson 216–24.
- Schaffer, 164; Uglow, 356; McEvoy (1983), 56–57; Donovan, 175–76, 180–81.
- See Schaffer, 162–70 for a historiographical analysis.
- Schofield (2004), 194.
- McEvoy (1983), 51ff.
- McEvoy (1983), 57; see also McEvoy and MeGuire 395ff.
- Qtd. in Thorpe, 213.
- Priestley, Joseph. An History of the Corruptions of Christianity. 2 vols. Birmingham: Printed by Piercy and Jones; London: Printed for J. Johnson, 1782.
- Schofield (2004), 216.
- Qtd. in Gibbs, 249.
- Schofield (2004), 216–23; Thorpe, 106–08; Holt, 133–39; Philip (1985).
- Priestley, Joseph. The importance and extent of free inquiry in matters of religion: a sermon, preached before the congregations of the Old and New Meeting of Protestant Dissenters at Birmingham. 5 November 1785. To which are added, reflections on the present state of free inquiry in this country. Birmingham: Printed by M. Swinney; for J. Johnson, London, 1785.
- Qtd. in Gibbs, 173.
- Gibbs, 169–76; Uglow, 408.
- Gibbs, 176–83.
- Priestley, Joseph. A letter to the Right Honourable William Pitt, … on the subjects of toleration and church establishments; occasioned by his speech against the repeal of the Test and Corporation Acts, on Wednesday 28 March 1787. London: Printed for J. Johnson and J. Debrett, 1787.
- Priestley, Joseph. Letters to the Right Honourable Edmund Burke, occasioned by his Reflections on the Revolution in France, &c. Birmingham: Printed by Thomas Pearson; sold by J. Johnson, London, 1791.
- Schofield (2004), 269–81; Thorpe, 122–25; Uglow, 409, 435–38; Holt, 142ff; Philip (1985).
- Qtd. in Crossland, 294.
- Crossland, 283–87, 305.
- Kramnick, 22.
- Page, Anthony (2011). “Rational dissent, enlightenment and abolition of the British slave trade”. The Historical Journal. 54 (3): 748–49. doi:10.1017/S0018246X11000227. S2CID 145068908.
- Dionisio, Jennifer (Summer 2010). “Birmingham Toast”. Chemical Heritage Magazine. 28 (2): 18.
- Qtd. in Gibbs, 204; Schofield (2004), 264, 285, 289; Thorpe, 122–44; Uglow, 440–46; Jackson, 248–60; Rose, 68–88; Holt, 154ff.
- Coleridge, Samuel Taylor. “Religious Musings: A Desultory Poem, Written on the Christmas Eve of 1794”. Archived from the original on 12 January 2010. Retrieved 1 January 2010.
- “Joseph Priestley at hackney.gov.uk”. Archived from the original on 3 March 2016. Retrieved 11 June 2010.
- Schaffer, 160; Schofield (2004), 298–99; Thorpe, 145–46; Uglow, 446–49; Jackson, 300–05.
- Priestley, Joseph. An Appeal to the Public on the Subject of the Riots in Birmingham. To which are added, strictures on a pamphlet, entitled ‘Thoughts on the late riot at Birmingham.‘ Birmingham: Printed by J. Thompson; sold by J. Johnson, London, 1791.
- Qtd. in Schofield (2004), 295.
- A blue plaque marks the site of the Gravel Pit Meeting at Ram Place and a brown plaque the site of the Priestleys’ house at 113, Lower Clapton Road: Joseph Priestley at hackney.gov.uk Archived 3 March 2016 at the Wayback Machine
- Garrett, 53, 57, 61.
- Qtd. in Garrett, 62.
- Schofield (2004), p. 318.
- Gibbs (1965), p. 214.
- Gibbs (1965), p. 216; Schofield (2004), p. 318.
- Graham (1995), p. 26.
- Gibbs (1965), p. 216; Schofield,(2004), p. 318.
- Schwartz, A. Truman; McEvoy, John G., eds. (1990). Motion toward perfection : the achievement of Joseph Priestley. Boston, Mass.: Skinner House Books. p. 199. ISBN 978-1558960107. Archived from the original on 3 June 2016. Retrieved 11 September 2014.
- Graham (1995), p. 27; Schofield (2004), p. 318.
- Graham (1995), p. 33.
- Graham (1995), p. 35.
- Gibbs, 207–22; Schofield (2004), 304–18; Thorpe, 145–55; Uglow, 446–49, 453–54; Jackson, 300–05; Holt, 177–78.
- Schofield (2004), 329–30.
- Schofield (2004), 324–32; Thorpe, 155–57; Jackson, 310–14; Holt, 179ff.
- Mary Cathryne Park, Joseph Priestley and the problem of Pantisocrasy (Philadelphia, 1947), 14–24, 52–57. Penn State University Library, The Joseph Priestley Collection. “The Joseph Priestley Collection”. Archived from the original on 24 June 2013. Retrieved 21 June 2013. Property inventory assets and debts account book, 1807–1810
- Tony Rail, “William Priestley vindicated,” Enlightenment and Dissent no.28 (2012); 150–195. “Dr Williams’s Centre for Dissenting Studies”. Archived from the original on 24 December 2013. Retrieved 23 December 2013.
- Tony Rail, op. cit. 161.
- Rutt, I(ii), 354.
- McLachlan (1983), 34.
- Schofield (2004), 326.
- Signed ‘A Quaker in politics,’ the Maxims were printed over two days in the Aurora General Advertiser, 26 & 27 February 1798, and reprinted in both the Auroraand Carey’s United States’ Recorder, 31 March & 1 April 1799. See Rutt, XXV, 175-82.
- Copies of original letters recently written by persons in Paris to Dr. Priestley in America, taken on board of a neutral vessel (London, 1798). Federal Gazette (Baltimore, MD), 27 August 1798.
- Vaughan had fled to France in May 1794, when John Hurford Stone’s brother, William, was arrested and found to have a letter from Vaughan. In France, to avoid arrest as an Englishmen, he assumed the name of Jean Martin, and lived quietly at Passy. (John G. Alger, Englishmen in the French Revolution (London, 1889), 93).
- Tony Rail, op. cit.; Schofield (2004), 329–38; Gibbs, 234–37; Jackson, 317–18; Garrett, 63; Holt, 199–204.
- In December 1799, two of Elizabeth Ryland-Priestley’s essays, On the propriety and expediency of unlimited enquiry, and A Reply to [Thomas Cooper’s] Observations on the Fast Day [Cooper had challenged the power of a President to declare a day of fasting and prayer], were published as part of Political essays (Northumberland, PA, 1799). [Eugene Volokh: “Elizabeth Ryland-Priestley, Early American author on free speech”; New York University Journal of Law & Liberty, 4(2) (2009), 382–5].
- Tony Rail, op. cit. 166–7.
- Published in two parts, Northumberland-town PA, 1799; printed by Andrew Kennedy who printed the Sunbury and Northumberland Gazette. A pirate edition seems to have been published at Albany NY for Samuel Campbell of New York. (Robert E Schofield, A scientific autobiography of Joseph Priestley (Cambridge, MS, 1966), 303).
- Dr Priestley suffered a bilious and bowel condition throughout his adult life, with episodes of severe diarrhoea, for which Margaret Foulke-Priestley seems to have suggested a diet that used maize flour (US Cornmeal), and excluded wheat flour. (Tony Rail, op. cit. 156, 161).
- Tony Rail, op. cit.
- Priestley, Joseph. A General History of the Christian Church. Northumberland: Printed for the author by Andrew Kennedy, 1803.
- Qtd. in Schofield (2004), 339–43.
- “APS Member History”. search.amphilsoc.org. Retrieved 16 December 2020.
- Schofield (2004), 352–72; Gibbs, 244–46.
- Schofield (2004), 400–01; Gibbs, 247–48; Thorpe, 162–65; Jackson, 324–25; Holt, 213–16.
- In accordance with known birth-death dates. His original headstone gives his age as “LXXI” (71).
- For the original marker, see “Edgar Fahs Smith Collection”. Archived from the original on 23 December 2008. Retrieved 17 November 2006. See also page 153 of Walker, William H. (1927). “History of the Priestley house and the movement for its preservation”. Journal of Chemical Education. 4 (2): 150–158. Bibcode:1927JChEd…4..150W. doi:10.1021/ed004p150
- Qtd. in Schofield (2004), 401.
- Schofield (2004), 151–52.
- Qtd. in McLachlan (1987–90), 259–60.
- Thorpe, 74; Kramnick, 4.
- Tapper, 322.
- Schofield (2004), 3.
- Schofield (2004), 52–57; Holt, 111–12.
- McEvoy (1983), 47.
- Schaffer, 154–57.
- Eshet, 131.
- “Joseph Priestley College”. Joseph Priestley College. Archived from the original on 14 November 2007. Retrieved 1 January 2010.
- Schmadel, Lutz D. Dictionary of Minor Planet Names. 5th ed. Berlin and New York: Springer (2003), 474.
- The statue in Birmingham is a 1951 recast, in bronze, of a white marble original by Francis John Williamson, unveiled on 1 August 1874.
- The Lunar Society Moonstones honour Priestley in Birmingham. There are Blue Plaques commemorating him on the side of the Church of St. Michael and St. Joseph, New Meeting House Lane, Birmingham (Birmingham Civic Society Archived 10 February 2008 at the Wayback Machine Retrieved 1 January 2010), and another on the Warrington Salvation Army Citadel, once the home of Priestley (British Crystallographic Association Archived 25 September 2006 at the Wayback Machine Retrieved 1 January 2010).
- “Minister opens £4m ‘state-of-the-art’ chemistry facilities at Leeds”, 20 October 2006, University of Leeds, Retrieved 25 November 2018.
- “University of Huddersfield”. www.hud.ac.uk. Archived from the original on 5 January 2017. Retrieved 8 February 2017.
- “Joseph Priestley Award”. Dickinson College. Retrieved 4 May 2021.
- “National Historic Chemical Landmarks”. Joseph Priestley and the Discovery of Oxygen. American Chemical Society. 2000. Archived from the original on 26 December 2015.
- Raber, Linda R. (7 April 2008). “85th Anniversary of the Priestley Medal”. Chemical & Engineering News. American Chemical Society. Retrieved 12 November 2018.
- “Priestley, James Taggart (1903–1979)”. Plarr’s Lives of the Fellows, Royal College of Surgeons of England.
- “UoB Calmview5: Search results”. calmview.bham.ac.uk. Retrieved 28 January 2021.
Bibliography
The most exhaustive biography of Priestley is Robert Schofield’s two-volume work; several older one-volume treatments exist: those of Gibbs, Holt and Thorpe. Graham and Smith focus on Priestley’s life in America and Uglow and Jackson both discuss Priestley’s life in the context of other developments in science.
- Gibbs, F. W. Joseph Priestley: Adventurer in Science and Champion of Truth. London: Thomas Nelson and Sons, 1965.
- Graham, Jenny. Revolutionary in Exile: The Emigration of Joseph Priestley to America, 1794–1804. Transactions of the American Philosophical Society 85 (1995). ISBN 0-87169-852-8.
- Holt, Anne (1970) [1931]. A life of Joseph Priestley. Westport, Conn., Greenwood Press. ISBN 978-0-8371-4240-1.
- Jackson, Joe. A World on Fire: A Heretic, an Aristocrat and the Race to Discover Oxygen. New York: Viking, 2005. ISBN 0-670-03434-7.
- Isaacson, Walter (2004). Benjamin Franklin : an American life. New York : Simon & Schuster. ISBN 978-0-6848-07614.
- Johnson, Steven. The Invention of Air: A Story of Science, Faith, Revolution, and the Birth of America. New York: Riverhead, 2008. ISBN 1-59448-852-5.
- Kramnick, Isaac (January 1986). “Eighteenth-Century Science and Radical Social Theory: The Case of Joseph Priestley’s Scientific Liberalism”. Journal of British Studies. Cambridge University Press on behalf of The North American Conference on British Studies. 25 (1): 1–30. doi:10.1086/385852. JSTOR 175609. S2CID 197667044.
- Schofield, Robert E. (1997). The enlightenment of Joseph Priestley : a study of his life and work from 1733 to 1773. University Park, Pa. : Pennsylvania State University Press. ISBN 978-0-2710-1662-7.
- Schofield, Robert E. (2004). The Enlightened Joseph Priestley: A Study of His Life and Work from 1773 to 1804. Penn State Press. ISBN 978-0-2710-3246-7.
- Smith, Edgar F. Priestley in America, 1794–1804. Philadelphia: P. Blakiston’s Son and Co., 1920.
- Tapper, Alan. “Joseph Priestley”. Dictionary of Literary Biography 252: British Philosophers 1500–1799. Eds. Philip B. Dematteis and Peter S. Fosl. Detroit: Gale Group, 2002.
- Thorpe, T. E. Joseph Priestley. London: J. M. Dent, 1906.
- Uglow, Jenny. The Lunar Men: Five Friends Whose Curiosity Changed the World. New York: Farrar, Straus and Giroux, 2002. ISBN 0-374-19440-8.
- Van Doren, Carl (1938). Benjamin Franklin. New York, Garden City Publishing Company.
Secondary materials
- Anderson, R. G. W. and Christopher Lawrence. Science, Medicine and Dissent: Joseph Priestley (1733–1804). London: Wellcome Trust, 1987. ISBN 0-901805-28-9.
- Bowers, J. D. Joseph Priestley and English Unitarianism in America. University Park: Pennsylvania State University Press, 2007. ISBN 0-271-02951-X.
- Braithwaite, Helen. Romanticism, Publishing and Dissent: Joseph Johnson and the Cause of Liberty. New York: Palgrave Macmillan, 2003. ISBN 0-333-98394-7.
- Conant, J. B., ed. “The Overthrow of the Phlogiston Theory: The Chemical Revolution of 1775–1789”. Harvard Case Histories in Experimental Science. Cambridge: Harvard University Press, 1950.
- Crook, R. E. A Bibliography of Joseph Priestley. London: Library Association, 1966.
- Crossland, Maurice. “The Image of Science as a Threat: Burke versus Priestley and the ‘Philosophic Revolution'”. British Journal for the History of Science 20 (1987): 277–307.
- Donovan, Arthur. Antoine Lavoisier: Science, Administration and Revolution. Cambridge: Cambridge University Press, 1996. ISBN 0-521-56218-X
- Eshet, Dan. “Rereading Priestley”. History of Science 39.2 (2001): 127–59.
- Fitzpatrick, Martin. “Joseph Priestley and the Cause of Universal Toleration”. The Price-Priestley Newsletter 1 (1977): 3–30.
- Garrett, Clarke. “Joseph Priestley, the Millennium, and the French Revolution”. Journal of the History of Ideas 34.1 (1973): 51–66.
- Fruton, Joseph S. Methods and Styles in the Development of Chemistry. Philadelphia: American Philosophical Society, 2002. ISBN 0-87169-245-7.
- Gray, Henry Colin; Harrison, Brian Howard (2004). Joseph Priestly. Vol. XLV. Oxford; New York : Oxford University Press: Oxford dictionary of national biography. pp. 351–359–.
- Kramnick, Isaac. “Eighteenth-Century Science and Radical Social Theory: The Case of Joseph Priestley’s Scientific Liberalism”. Journal of British Studies 25 (1986): 1–30.
- Kuhn, Thomas. The Structure of Scientific Revolutions. 3rd ed. Chicago: University of Chicago Press, 1996. ISBN 0-226-45808-3.
- Haakonssen, Knud, ed. Enlightenment and Religion: Rational Dissent in Eighteenth-Century Britain. Cambridge: Cambridge University Press, 1996. ISBN 0-521-56060-8.
- McCann, H. Chemistry Transformed: The Paradigmatic Shift from Phlogiston to Oxygen. Norwood: Alex Publishing, 1978. ISBN 0-89391-004-X.
- McEvoy, John G. “Joseph Priestley, ‘Aerial Philosopher’: Metaphysics and Methodology in Priestley’s Chemical Thought, from 1762 to 1781”. Ambix 25 (1978): 1–55, 93–116, 153–75; 26 (1979): 16–30.
- McEvoy, John G. “Enlightenment and Dissent in Science: Joseph Priestley and the Limits of Theoretical Reasoning”. Enlightenment and Dissent 2 (1983): 47–68.
- McEvoy, John G. “Priestley Responds to Lavoisier’s Nomenclature: Language, Liberty, and Chemistry in the English Enlightenment”. Lavoisier in European Context: Negotiating a New Language for Chemistry. Eds. Bernadette Bensaude-Vincent and Ferdinando Abbri. Canton, MA: Science History Publications, 1995. ISBN 0-88135-189-X.
- McEvoy, John G. and J.E. McGuire. “God and Nature: Priestley’s Way of Rational Dissent”. Historical Studies in the Physical Sciences 6 (1975): 325–404.
- McLachlan, John. Joseph Priestley Man of Science 1733–1804: An Iconography of a Great Yorkshireman. Braunton and Devon: Merlin Books, 1983. ISBN 0-86303-052-1.
- McLachlan, John. “Joseph Priestley and the Study of History”. Transactions of the Unitarian Historical Society 19 (1987–90): 252–63.
- Philip, Mark. “Rational Religion and Political Radicalism”. Enlightenment and Dissent 4 (1985): 35–46.
- Rose, R. B. “The Priestley Riots of 1791”. Past and Present 18 (1960): 68–88.
- Rosenberg, Daniel. Joseph Priestley and the Graphic Invention of Modern Time. Studies in Eighteenth Century Culture 36(1) (2007): pp. 55–103.
- Rutherford, Donald. Leibniz and the Rational Order of Nature. Cambridge: Cambridge University Press, 1995. ISBN 0-521-46155-3.
- Schaffer, Simon. “Priestley Questions: An Historiographic Survey”. History of Science 22.2 (1984): 151–83.
- Sheps, Arthur. “Joseph Priestley’s Time Charts: The Use and Teaching of History by Rational Dissent in late Eighteenth-Century England”. Lumen 18 (1999): 135–54.
- Watts, R. “Joseph Priestley and Education”. Enlightenment and Dissent 2 (1983): 83–100.
Primary materials
- Lindsay, Jack, ed. Autobiography of Joseph Priestley. Teaneck: Fairleigh Dickinson University Press, 1970. ISBN 0-8386-7831-9.
- Miller, Peter N., ed. Priestley: Political Writings. Cambridge: Cambridge University Press, 1993. ISBN 0-521-42561-1.
- Passmore, John A., ed. Priestley’s Writings on Philosophy, Science and Politics. New York: Collier Books, 1964.
- Rutt, John T., ed. Collected Theological and Miscellaneous Works of Joseph Priestley. Two vols. London: George Smallfield, 1832.
- Rutt, John T., ed. Life and Correspondence of Joseph Priestley. Two vols. London: George Smallfield, 1831.
- Schofield, Robert E., ed. A Scientific Autobiography of Joseph Priestley (1733–1804): Selected Scientific Correspondence. Cambridge: MIT Press, 1966.
- https://en.wikipedia.org/wiki/Phlogiston_theory
- https://en.wikipedia.org/wiki/Johann_Heinrich_Pott
- White, John Henry (1973). The History of Phlogiston Theory. New York: AMS Press Inc. ISBN 978-0404069308.
- Klein, Ursula (2004-11-05). “Not a Pure Science: Chemistry in the 18th and 19th Centuries”. Science. 306 (5698): 981–982. doi:10.1126/science.1089888. ISSN 0036-8075. S2CID 10923830.
- Cassebaum, Heinz (1979). “Die Stellung der Braunstein-Untersuchungen von J. H. Pott (1692-1777) in der Geschichte des Mangans”. Sudhoffs Archiv. 63 (2): 136–153. ISSN 0039-4564. JSTOR 20776589.
- Engel, Michael (2001). “Pott, Johann Heinrich”. Neue Deutsche Biographie (in German). Vol. 20. Berlin: Duncker & Humblot. p. 660.
- Hufbauer, Karl (1982). The Formation of the German Chemical Community, 1720-1795. University of California Press. pp. 176–177.
- Greenaway, Frank (1975). “Johann Heinrich Pott”. Dictionary of scientific biography. Vol. 11. p. 109.
- https://en.wikipedia.org/wiki/Johann_Juncker
- https://en.wikipedia.org/wiki/Francke_Foundations
- Fichman, Martin. “Juncker, Johann”. Complete Dictionary of Scientific Biography. Encyclopedia.com. Retrieved 1 November 2017.
- Hufbauer, Karl (1982). The formation of the German chemical community, 1720-1795. Berkeley u.a.: University of California Press. pp. 172–173. ISBN 978-0-520-04318-3. Retrieved 1 November 2017.
- Wilson, Renate (2003). “Chapter 8: Pietist Universal Reform and care of the sick and the poor”. In Finzsch, Norbert; Jütte, Robert (eds.). Institutions of confinement : hospitals, asylums, and prisons in Western Europe and North America, 1500-1950 (1st. paperback ed.). Cambridge: Cambridge University Press. p. 134. ISBN 9780521534482. Retrieved 2 November 2017.
- “Johann Juncker”. Martin Luther Universitat Halle Wittenberg. Retrieved 1 November 2017.
- Zumkeller, W (2001). “The University of Halle through the centuries”. Molecular Pathology. 54 (1): 36–37. doi:10.1136/mp.54.1.36-a. PMC 1187002. PMID 11212887.
- “Halle Pietism and Reformation”. Franckesche Stiftungen zu Halle. Retrieved 1 November 2017.
- Dorwart, Reinhold August (1971). The Prussian welfare state before 1740. Cambridge, MA: Harvard University Press. p. 208. ISBN 9780674330283.
- Bynum, W. F.; Porter, Roy (1985). William Hunter and the eighteenth-century medical world (Paperback ed.). Cambridge: Cambridge University Press. pp. 192–194. ISBN 978-0521268066. Retrieved 2 November 2017.
- Westfall, Richard S. “Juncker, Johann”. The Galileo Project. Retrieved 1 November 2017.
- Granquist, Mark A.; Haemig, Mary Jane; Kolb, Robert; Mattes, Mark C.; Strom, Jonathan (August 22, 2017). Dictionary of Luther and the Lutheran Traditions. Baker Academic. ISBN 9780801049699. Retrieved 2 November 2017.
- Gawthrop, Richard L. (2006). Pietism and the making of eighteenth-century Prussia (1st pbk. ed.). Cambridge: Cambridge Univ. Press. pp. 171–173. ISBN 9780521030120.
- “Francke, August Hermann” . Encyclopædia Britannica. Vol. 11 (11th ed.). 1911. p. 4.
- Wilson, Renate (2000). Pious traders in medicine : a German pharmaceutical network in eighteenth-century North America. University Park, Pa.: Pennsylvania State University Press. pp. 43–65. ISBN 9780271028330. Retrieved 2 November 2017.
- Stahl, Georg Ernst (1859). Oeuvres M Dico-Philosophiques Et Pratiques. Vol. 2. Paris: T. Blondin J. -B. Baillire et fils. p. 137. ISBN 9781271855896. Retrieved 2 November 2017.
- Winn, Christian T. Collins; Carlson, G. William; Gehrz, Christopher; Holst, Erich, eds. (2011). The pietist impulse in Christianity. Eugene, Ore.: Pickwick. p. 299. ISBN 978-1606083277. Retrieved 2 November 2017.
- Broman, Thomas H. (1996). The transformation of German academic medicine, 1750-1820 (1st ed.). Cambridge: Cambridge Univ. Press. pp. 62–65. ISBN 9780521552318. Retrieved 1 November 2017.
- Lindemann, Mary (2010). Medicine and Society in Early Modern Europe (2nd ed.). Cambridge: Cambridge University Press. p. 146. ISBN 9780521732567. Retrieved 2 November 2017.
- Ricci, James V. (1990). The development of gynæcological surgery and instruments. San Francisco, Calif.: Norman Pub. p. 253. ISBN 9780930405281. Retrieved 1 November 2017.
- Juncker, Johann (1721). Conspectus chirurgiae : tam medicae, methodo Stahliana conscriptae, quam instrumentalis, recentissimorum auctorum ductu collectae : quae singula tabulis CIII exhibentur : adjecto indice sufficiente. Halae: Typis & impensis Orphanotrophei.
- “Juncker, Johann 1679-1759”. WorldCat Identities. Retrieved 1 November 2017.
- Dahl, Gina (2011). Books in early modern Norway. Leiden: Brill. pp. 127–128. ISBN 9789004207202. Retrieved 2 November 2017.
- Spary, E. C. (2014). Feeding France New Sciences of Food, 1760-1815. Cambridge Univ Pr. p. 91. ISBN 9781107031050. Retrieved 2 November 2017.
- Watkins, Eric, ed. (2001). Kant and the sciences. Oxford: Oxford University Press. p. 218. ISBN 978-0195133059.
- “Ortsfamilienbuch Gießen Familienbericht”. Genealogy.net. Retrieved 2 November 2017.
- https://en.wikipedia.org/wiki/Phlogiston_theory
- https://en.wikipedia.org/wiki/Guillaume-Fran%C3%A7ois_Rouelle
- Dorveaux, Paul (1933). “Apothicaires membres de l’Académie Royale des sciences : IX. Guillaume-François Rouelle”. Revue d’Histoire de la Pharmacie (in French). 84 (December): 169–186. doi:10.3406/pharm.1933.10035. Retrieved 24 March 2019.
- Chisholm, Hugh, ed. (1911). “Rouelle, Guillaume François“. Encyclopædia Britannica. Vol. 23 (11th ed.). Cambridge University Press. p. 768.
- Jensen, William B. (2006). “The origin of the term “base””. The Journal of Chemical Education. 83 (8): 1130. Bibcode:2006JChEd..83.1130J. doi:10.1021/ed083p1130.
- Source https://www.physicsforums.com/threads/etymology-of-base.543937/
One or more of the preceding sentences incorporates text from a publication now in the public domain:
Further reading
- Franckowiak, Rémi (2003). “Rouelle, un vrai-faux anti-newtonien”. Archives internationales d’histoire des sciences. 53 (150–151): 240–255.
- Franckowiak, Rémi (2002). “Les sels neutres de Guillaume-François Rouelle” (PDF). Revue d’Histoire des Sciences. 55 (4): 493–532. doi:10.3406/rhs.2002.2163.
- Lemay, Pierre; Oesper, Ralph E. (1954). “The lectures of Guillaume Francois Rouelle”. The Journal of Chemical Education. 31 (7): 338. Bibcode:1954JChEd..31..338L. doi:10.1021/ed031p338.
- Warolin, C. (1996). “Did Lavoisier benefit from the teachings of the apothecary Guillaume-François Rouelle?”. Histoire des sciences médicales. 30 (1): 30. PMID 11624829.
- Warolin, C. (1995). “Did Lavoisier benefit from the teachings of the apothecary Guillaume-François Rouelle?”. Revue d’histoire de la pharmacie. 42 (307): 361–367. doi:10.3406/pharm.1995.4257. PMID 11624913.
- https://en.wikipedia.org/wiki/Phlogiston_theory
- https://en.wikipedia.org/wiki/Giovanni_Antonio_Giobert
- Abbri, Ferdinando (2001). “GIOBERT, Giovanni Antonio”. Dizionario Biografico degli Italiani. 55. Retrieved 15 September 2017.
- Ronalds, Sir Francis (1880). Catalogue of Books and Papers Relating to Electricity. London: E. & F. N. Spon. p. 202. Retrieved 14 September 2017.
- Royal Society of London (1868). Catalogue of Scientific Papers (1800-1863). Vol. II. London: George Edward Eyre & William Spottiswoode. pp. 892–893. Retrieved 15 September 2017.
- Benedict, Francis Gano (1912). The Composition of the Atmosphere with Special Reference to Its Oxygen Content. Washington, D.C.: Carnegie Institution of Washington. p. 23. ISBN 9780608062167. Retrieved 18 September 2017.
- “Progress of Manufactures, Science, and the Fine Arts”. The Scots Magazine and Edinburgh Literary Miscellany. Archibald Constable & Company. 67: 367. 1805.
- “Galvanism”. Encyclopaedia Britannica: Or, A Dictionary of Arts, Sciences, and Miscellaneous Literature, Enlarged and Improved. Vol. 9 (6 ed.). Edinburgh: Archibald Constable & Company. 1823. p. 366.
- Rees, Abraham (1819). “Magnesite”. The cyclopædia; or, Universal dictionary of arts, sciences, and literature, Volume 22: MAC-MAT. London: Longman, Hurst, Rees, Orme & Brown. Retrieved 18 September 2017.
- “Giobert, (il cavaliere Giovanni Antonio)”. Biografia universale antica e moderna. Supplemento. Vol. IX. Venezia: Presso Giambattista Missiaglia. 1841. pp. 100–104. Retrieved 19 September 2017.
- “L’università di Asti ricorda Giobert: la fotogallery”. Gazzetta D’Asti. 21 ottobre 2013. 2013-10-20. Retrieved 18 September 2017.
- Accademia delle Scienze (1883). Il Primo Secolo Della R. Accademia Delle Scienze Di Torino. Notizie Storiche E Bibliografice. (1783-1883.). Turin: Stamperia Reale de G. B. Paravia E. C. p. 257. Retrieved 18 September 2017.
- Robinson, G. (1792). “Foreign Literature of the Year 1791”. The New Annual Register: Or General Repository of History, Politics, and Literature for the Year 1791. London: G. G. J. and J. Robinson. p. 300.
- Robinson, G. (1790). “Foreign literature of the year 1789”. The New Annual Register: Or General Repository of History, Politics, Arts, Sciences, and Literature. 32: 302. Retrieved 14 September 2017.
- Poggendorff, J. C.; Sächsische Akademie der Wissenschaften zu Leipzig (1863). Biographisch-literarisches Handwörterbuch der exakten Naturwissenschaften. Berlin: Akademie-Verlag.
Giobert.
- “Prof. Dr. Giovanni Antonio Giobert”. Bayerische Akademie der Wissenschaften. Retrieved 18 September 2017.
- “Muriatic acid”. Retrospect of Philosophical, Mechanical, Chemical, and Agricultural Discoveries. London: J. Wyatt. 1806. p. 183. Retrieved 19 September 2017.
- Meyer, Ernst von; McGowan, George (1906). A history of chemistry from earliest times to the present day; being also an introduction to the study of the science. London: Macmillan. p. 176. ISBN 978-1344996976. Retrieved 18 September 2017.
- Magiels, Geerdt (2010). From Sunlight to Insight : Jan IngenHousz, the Discovery of Photosynthesis (A crucial instrument: the rise and fall of the eudiometer ed.). Bruxelles: ASP. p. 225. ISBN 9789054876458. Retrieved 14 September 2017.
- Saussure, Theodore de; Hill, Jane F. (2012). Chemical research on plant growth. New York: Springer. p. ix. ISBN 9781461441359. Retrieved 18 September 2017.
- “Art. V. The American Mineralogical Journal”. Edinburgh Review, or Critical Journal for Nov. 1810-Feb. 1811. Edinburgh: D. Willison. 17: 119. 1811. Retrieved 18 September 2017.
- Mellor, Joseph William (1947). A Comprehensive Treatise on Inorganic and Theoretical Chemistry. Vol. 6. London: Longmans, Green. p. 427.
- Guyton, M. (1807). “Extract from Essays, by M. Jousselin, on the improvement of pottery…” (PDF). The Repertory of Arts, Manufactures, and Agriculture. 2. London: Printed for J. Wyatt. 11: 398–397. Retrieved 19 September 2017.
- Morton, W. J. T.; Simonds, James Beart (1859). “The Food of Plants”. The Veterinarian. London: Longmans, Green, Longmans & Roberts. XXXII (373): 526. Retrieved 19 September 2017.
- Nieto-Galan, Agustí (2001). Colouring Textiles A History of Natural Dyestuffs in Industrial Europe. Dordrecht: Springer Netherlands. ISBN 978-94-017-1081-7. Retrieved 14 September 2017.
- Watts, Henry (1874–77). “Indigo-Blue”. A dictionary of chemistry and the allied branches of other sciences. London: Longmans, Green, and co. p. 250.
- Watson, John Forbes (1873). Vienna Universal Exhibition, 1873. A classified and descriptive Catalogue of the Indian Department. London: W. H. Allen & Co. p. 115. Retrieved 19 September 2017.
- Predari, Francesco (1865). “Giobert (Cav.) (Giovanni Antonio)”. Dizionario biografico universale. Vol. 1. Milano: Tipografia Guigoni. p. 620. Retrieved 18 September 2017.
- Netz, Reviel; Noel, William (2007). The Archimedes codex : how a medieval prayer book is revealing the true genius of antiquity’s greatest scientist (1st Da Capo Press ed.). Philadelphia, PA: Da Capo Press. pp. 160–161. ISBN 978-0306815805. Retrieved 19 September 2017.
- Ripley, George; Dana, Charles A. (1874). “Gioberti, Giovanni Antonio”. The American Cyclopaedia: A Popular Dictionary of General Knowledge. Vol. 7. New York: Appleton. p. 816. Retrieved 19 September 2017.
- Begni, Ernesto; Grey, James C.; Kennedy, Thomas J. (1914). The Vatican : its history–its treasures. New York: Letters and Arts Pub. Co. pp. 431–432. Retrieved 19 September 2017.
- Morselli, Mario (1984). Amedeo Avogadro, a scientific biography. Dordrecht: D. Reidel Pub. Co. pp. 24–25, 29. ISBN 9789027716248. Retrieved 14 September 2017.
- Prospetto del Giornale Intitulato il Repubblicano Piemontese. Dalla Stamperia Mairese. 1798. p. 10.
- Woodward, Bernard Bolingbroke; Cates, William Leist Readwin (1872). “Gioberti, Giovanni Antonio”. Encyclopedia of Chronology: Historical and Biographical. London: Longmans, Green and Company. p. 593. Retrieved 19 September 2017.
- “Giovanni Antonio Giobert”. Comune di MONGARDINO. Retrieved 19 September 2017.
- “La figura di Giovanni Giobert in un convegno all’Università di Asti”. Archivio ATnews. 11 October 2013. Retrieved 20 September 2017.
External links
- “Giobert, Giovanni Antonio”. Smithsonian Libraries. Retrieved 14 September 2017.
British chemist Elizabeth Fulhame demonstrated through experiment that many oxidation reactions occur only in the presence of water, that they directly involve water, and that water is regenerated and is detectable at the end of the reaction. Based on her experiments, she disagreed with some of the conclusions of Lavoisier as well as with the phlogiston theorists that he critiqued. Her book on the subject appeared in print soon after Lavoisier’s execution for Farm-General membership during the French Revolution.
- Rayner-Canham, Marelene; Rayner-Canham, Geoffrey (2001). Women in chemistry: their changing roles from alchemical times to the mid-twentieth century. Philadelphia: Chemical Heritage Foundation. pp. 28–31. ISBN 978-0941901277. Retrieved 2 March 2016.
- Datta, N. C. (2005). The story of chemistry. Hyderabad: Universities Press. pp. 247–250. ISBN 9788173715303. Retrieved 2 March 2016.
- Wikipedia https://en.wikipedia.org/wiki/Phlogiston_theory
Biography
Elizabeth Fulhame (fl. 1794) was an early British chemist who invented the concept of catalysis and discovered photoreduction. She was described as ‘the first solo woman researcher of modern chemistry’.
Although she only published one text, she describes catalysis as a process at length in her 1794 book An Essay On Combustion with a View to a New Art of Dying and Painting, wherein the Phlogistic and Antiphlogistic Hypotheses are Proved Erroneous. The book relates in painstaking detail her experiments with oxidation-reduction reactions, and the conclusions she draws regarding phlogiston theory, in which she disagrees with both the Phlogistians and Antiphlogistians.
In 1798, the book was translated into German by Augustin Gottfried Ludwig Lentin as Versuche über die Wiederherstellung der Metalle durch Wasserstoffgas. In 1810, it was published in the United States, to much critical acclaim. That same year, Fulhame was made an honorary member of the Philadelphia Chemical Society. Thomas P. Smith applauded her work, stating that “Mrs. Fulhame has now laid such bold claims to chemistry that we can no longer deny the sex the privilege of participating in this science also.”
Personal life
Elizabeth Fulhame published under her married name, as Mrs. Fulhame. She was married to Thomas Fulhame, an Irish-born physician who had attended the University of Edinburgh and studied puerperal fever as a student of Andrew Duncan (1744–1828). Dr Thomas Fulhame was listed in Edinburgh directories between 1784–1800 (Bristo Square in 1784, Bristo Street in 1794, at 9 Society 1799, in Brown’s Square 1800). She is believed by some to have been Scottish, but the evidence for this seems to be little more than that her husband studied in Edinburgh — on that basis Charles Darwin’s wife Emma could be claimed as Scottish, but she clearly was not. Sir Benjamin Thompson, Count Rumford, referred to her as “the ingenious and lively Mrs. Fulhame”, however this opinion may reflect the style of her book.
Work

Mrs. Fulhame’s work began with her interest in finding a way of staining cloth with heavy metals under the influence of light. She originally considered calling her work An Essay on the Art of making Cloths of Gold, Silver, and other Metals, by chymical processes, but considering the “imperfect state of the art”, decided to select a title reflecting the broader implications of her experiments.: viii–ix
“The possibility of making cloths of gold, silver, and other metals, by chymical processes, occurred to me in the year 1780: the project being mentioned to Doctor Fulhame, and some friends, was deemed improbable. However, after some time, I had the satisfaction of realizing the idea, in some degree, by experiment.”:
She was apparently encouraged to publish an account of her 14 years of research as a result of meeting Sir Joseph Priestley in 1793. Fulhame studied the experimental reduction of metallic salts in a variety of states (aqueous solution, dry state, and sometimes an ether or alcohol solution) by exposing them to the action of various reducing agents. The metal salts she examined included gold, silver, platinum, mercury, copper, and tin. As reducing agents, she experimented with hydrogen gas, phosphorus, potassium sulfide, hydrogen sulfide, phosphine, charcoal, and light. She discovered a number of chemical reactions by which metal salts could be reduced to pure metals. Rayner-Canham considers her most important contribution to chemistry to be the discovery that metals could be processed through aqueous chemical reduction at room temperature, as an alternative to smelting at high temperatures.
Her theoretical work on catalysis was “a major step in the history of chemistry”, predating both Jöns Jakob Berzelius and Eduard Buchner. She proposed, and demonstrated through experiment, that many oxidation reactions occur only in the presence of water, that they directly involve water, and that water is regenerated and is detectable at the end of the reaction. Further, she proposed “recognisably modern mechanisms” for those reactions, and may have been the first scientist to do so. The role of oxygen, as she describes it, differs significantly from other theories of the time. Based on her experiments, she disagreed with some of the conclusions of Antoine Lavoisier as well as with the phlogiston theorists that he critiqued. Her research could be seen as a precursor to the work of Jöns Jakob Berzelius, however Fulhame focused specifically on water rather than heavy metals.
Further, Eder, in 1905, and Schaaf consider her work on silver chemistry to be a landmark in the birth and early history of photography. Fulhame’s work on the role of light sensitive chemicals (silver salts) on fabric, predates Thomas Wedgwood’s more famous photogram trials of 1801. Fulhame did not, however, attempt to make “images” or representational shadow prints in the way Wedgwood did, but she did engage in photoreduction using light.
Reception
In addition to her book being republished in Germany and America, Fulhame’s experiments were reviewed in a French journal, and several British magazines, and were positively commented on by Sir Benjamin Thompson, Count Rumford, and Sir John Herschel.
According to the introduction of her book by her American editor in 1810, her work was lesser known than it could or should have been, adding that “the pride of science, revolted at the idea of being taught by a female”.
Fulhame says as much in her own preface to the work:
“But censure is perhaps inevitable: for some are so ignorant, that they grow sullen and silent, and are chilled with horror at the sight of anything that nears the semblance of learning, in whatever shape it may appear; and should be the spectre appear in the shape of a woman, the pangs which they suffer are truly dismal.”
Such a reaction, she says, was particularly acute amongst some who held esteemed positions, whom she described as having a ‘dictatorship in science’.
Fulhame published her experiments on reductions using water with metals in a book in the first place in order not to be “plagiarized.” She also describes her book as possibly serving as “a beacon to future mariners” (e.g. women) taking up scientific inquiries. Antoine Lavoisier was executed six months before the publication of her book and thus could not respond to her theory. Irish chemist William Higgins complained that she had ignored his work on the involvement of water in the rusting of iron, but magnanimously concluded “I read her book with great pleasure, and heartily wish that her laudible example may be followed by the rest of her sex.”
Fulhame’s work was largely forgotten by the end of the 19th century, but it was rediscovered by J. W. Mellor. In the 20th century, she was noted in Physics Today, as being the first to ‘systematically’ vary ‘her reaction conditions’ and to ‘generalise a whole class of reactions…. the reduction of metals’ and first to suggest an explanation for the situations where ‘water dissociated into its ionic form, facilitated the intermediate reaction steps, and was regenerated by the end of the metal reduction.’
See also
References
- Rayner-Canham, Marelene; Rayner-Canham, Geoffrey (2001). Women in chemistry : their changing roles from alchemical times to the mid-twentieth century. Philadelphia: Chemical Heritage Foundation. pp. 28–31. ISBN 978-0941901277. Retrieved 2 March 2016.
- “The mysterious case of Elizabeth Fulhame, a chemist and true pioneer of science”. The National. 31 January 2023. Retrieved 6 February 2023.
- Burwick, Frederick; Goslee, Nancy Moore; Hoeveler, Diane Long, eds. (2012). The encyclopedia of Romantic literature. Chichester, West Sussex [England]: Wiley-Blackwell. ISBN 9781405188104. Archived from the original on 7 March 2016. Retrieved 2 March 2016.
- Ogilvie, Marilyn Bailey (1986). Women in Science: Antiquity through the Nineteenth Century (4th print. ed.). Cambridge, Mass.: MIT Press. pp. 28–31. ISBN 978-0-262-65038-0.
- “Celebrating Scottish women of science”. National Library of Scotland. Archived from the original on 15 July 2017. Retrieved 20 June 2017.
- “Elizabeth Fulhame”. The Human Touch of Chemistry. Archived from the original on 3 March 2016. Retrieved 2 March 2016.
- Cornish-Bowden, Athel (2012). Fundamentals of enzyme kinetics. Weinheim: Wiley-VCH. ISBN 9783527665495. Retrieved 2 March 2016.
- Fulhame, T. (1784). Dissertatio de febre puerperarum. Academiæ Edinburgenæ, facultatis medicæ; pro gradu doctoris. Thomas Fulhame, M.A. hibernus. Ad diem 13. Septemb. Edinburgi: Apud Balfour et Smellie, academiae typographos. University of Edinburgh, Centre for Research Collections.
- “(456) – Towns > Edinburgh > 1773–1776, 1784–1785 – Williamson’s directory for the City of Edinburgh, Canongate, Leith and suburbs > 1784–85 – Scottish Directories – National Library of Scotland”. digital.nls.uk. Retrieved 10 February 2020.
- “(88) – Towns > Edinburgh > 1794–1795 – Directory for Edinburgh Leith Mussleburgh and Dalkeith – Scottish Directories – National Library of Scotland”. digital.nls.uk. Retrieved 10 February 2020.
- “(146) – Towns > Edinburgh > 1799–1800 – Edinburgh and Leith directory, to July 1800 – Scottish Directories – National Library of Scotland”. digital.nls.uk. Retrieved 10 February 2020.
- “(109) – Towns > Edinburgh > 1800–01 – Edinburgh and Leith directory to July 1801 – Scottish Directories – National Library of Scotland”. digital.nls.uk. Retrieved 10 February 2020.
- Ewan, Elizabeth L., ed. (2006). The biographical dictionary of Scottish women : from the earliest times to 2004. Edinburgh: Edinburgh Univ. Press. p. 130. ISBN 9780748617135. Retrieved 2 March 2016.
- Rumford, Benjamin, Graf von (1875). The complete works of Count Rumford. Vol. 4. Boston: American Academy of Arts and Sciences. p. 84. Retrieved 2 March 2016.
- Fulhame, Elizabeth (1794). An essay on combustion, with a view to a new art of dying and painting. Wherein the phlogistic and antiphlogistic hypotheses are proven erroneous. London: Printed for the author, by J. Cooper. Retrieved 2 March 2016.|
- Batchen, Geoffrey (1997). Burning with desire : the conception of photography (First MIT Press paperback ed.). Cambridge, Mass.: MIT Press. p. 28. ISBN 9780262024273.
- Davenport, Derek; Ireland, Kathleen (1989). “The Ingenious, Lively and Celebrated Mrs. Fulhame and the Dyer’s Hand” (PDF). Bulletin for the History of Chemistry (5): 37–42. Retrieved 2 March 2016.
- Laidler, Keith J.; Cornish-Bowden, Athel (1997). “”Elizabeth Fulhame and the discovery of catalysis: 100 years before Buchner” (PDF). In Cornish-Bowden, Athel (ed.). New beer in an old bottle : Eduard Buchner and the growth of biochemical knowledge. Valencia: Universitat de Valencia. pp. 123–126. ISBN 9788437033280. Archived from the original (PDF) on 23 January 2015. Retrieved 2 March 2016.
- Datta, N. C. (2005). The story of chemistry. Hyderabad: Universities Press. pp. 247–250. ISBN 9788173715303. Retrieved 2 March 2016.
- Eder, Josef Maria (1905). Geschichte der Photographie (in German) (3rd ed.). Halle: Wilhelm Knapp. OCLC 494529056.
- Laidler, Keith J. (1993). To Light such a Candle. Oxford University Press. pp. 68–69.
- Schaaf, Larry J. (1990). “The first fifty years of British photography, 1794–1844”. In Pritchard, Michael (ed.). Technology and art: the birth and early years of photography: the proceedings of the Royal Photographic Historical Group conference 1–3 September 1989. Bath: RPS Historical Group. pp. 9–18. ISBN 9780951532201.
- Schaaf, Larry J. (1992). Out of the shadows: Herschel, Talbot, & the invention of photography. New Haven: Yale University Press. pp. 23–25. ISBN 9780300057058.
- “The Woman Behind Photography”. Sotheby’s Institute of Art. Retrieved 8 March 2016.
- Fulhame, Elizabeth (1810). An essay on combustion, with a view to a new art of dying and painting : wherein the phlogistic and antiphlogistic hypotheses are proved erroneous. Philadelphia: Printed and sold by James Humphreys, Corner of Second and Walnut-streets. p. iv. Retrieved 20 June 2017.
- Linker, Jessica C. (April 2015). “The Pride of Science: Women and the politics of inclusion in 19th century Philadelphia” (PDF). Pennsylvania Legacies. 15 (1): 6–11. doi:10.5215/pennlega.15.1.0006. Retrieved 2 March 2016.
- Mellor, J. W. (1903). “History of the water problem (Mrs Fulhame’s theory of catalysis)”. Journal of Physical Chemistry. 7 (8): 557–567. doi:10.1021/j150053a001.
- Wikipedia https://en.wikipedia.org/wiki/Elizabeth_Fulhame
- https://en.wikipedia.org/wiki/Phlogiston_theory
- https://en.wikipedia.org/wiki/Torbern_Bergman
- “Chemistry”, Encyclopedia Britannica, 1911
- Chisholm 1911.
- “Library and Archive Catalog”. Royal Society. Retrieved 13 December 2010.[permanent dead link]
- “APS Member History”
- Membres de l’académie du passé
- Hamlin, Christopher. (1990) ‘A Science of Impurity, water analysis in nineteenth century Britain’, University of California Press
- Rines, George Edwin, ed. (1920). “Bergman, Torbern Olof” . Encyclopedia Americana.
- Chisholm, Hugh, ed. (1911). “Bergman, Torbern Olof“. Encyclopædia Britannica. Vol. 3 (11th ed.). Cambridge University Press. p. 774.
Further reading
- Mostrom, Birgitta. (1957). Torbern Bergman: a bibliography of his works. Stockholm: Almqvist & Wiksell. Includes over 300 items, including translations printed up to 1956.
- Schufle, J.A. (1985). Torbern Bergman : a man before his time. Lawrence, Kan.: Coronado Press.
- Smeaton, W.A. (1970). “Bergman, Torbern Olaf”. Dictionary of Scientific Biography. Vol. 2. New York: Charles Scribner’s Sons. ISBN 0-684-10114-9.
- Johannes Uray, Chemische Theorie und mineralogische Klassifikationssysteme von der chemischen Revolution bis zur Mitte des 19. Jahrhunderts. In: Berhard Hubmann, Elmar Schübl, Johannes Seidl (eds.), Die Anfänge geologischer Forschung in Österreich. Beiträge zur Tagung „10 Jahre Arbeitsgruppe Geschichte der Erdwissenschaften Österreichs” von 24. bis 26. April 2009 in Graz. Graz 2010, S 107–125.
External links
- Torbern Bergman Biography – by James S. Aber
- Bergman, Torbern (1735–1784). Opuscula Physica et Chemica. 6 vols. Uppsala, 1780.
- Bergman’s chemical genealogy
- Physisk Beskrifning öfver Jord-Klotet (2 volumes, 1773) – full digital facsimile from Linda Hall Library
Tab Content

In 1667, Johann Joachim Becher published his book Physica subterranea, which contained the first instance of what would become the phlogiston theory. In his book, Becher eliminated fire and air from the classical element model and replaced them with three forms of the earth: terra lapidea, terra fluida, and terra pinguis. Terra pinguis was the element that imparted oily, sulphurous, or combustible properties. Becher believed that terra pinguis was a key feature of combustion and was released when combustible substances were burned. Becher did not have much to do with phlogiston theory as we know it now, but he had a large influence on his student Stahl. Becher’s main contribution was the start of the theory itself, however much of it was changed after him. Becher’s idea was that combustible substances contain an ignitable matter, the terra pinguis.
- Bowler, Peter J (2005). Making modern science: A historical survey. Chicago: University of Chicago Press. p. 60. ISBN 9780226068602.
- Becher, Physica Subterranea p. 256 et seq.
- Brock, William Hodson (1993). The Norton history of chemistry (1st American ed.). New York: W. W. Norton. ISBN 978-0-393-03536-0.
- White, John Henry (1973). The History of Phlogiston Theory. New York: AMS Press Inc. ISBN 978-0404069308.
- Leicester, Henry M.; Klickstein, Herbert S. (1965). A Source Book in Chemistry. Cambridge, Massachusetts: Harvard University Press.
- Wikipedia https://en.wikipedia.org/wiki/Phlogiston_theory
Johann Joachim Becher (6 May 1635 – October 1682) was a German physician, alchemist, precursor of chemistry, scholar, polymath and adventurer, best known for his development of the phlogiston theory of combustion, and his advancement of Austrian cameralism.
Early life and education
Becher was born in Speyer during the Thirty Years War. His father was a Lutheran minister and died when Becher was a child. At the age of thirteen Becher found himself responsible not only for his own support but also for that of his mother and two brothers. He learned and practiced several small handicrafts, devoted his nights to study of the most miscellaneous description and earned a pittance by teaching.
In 1654, at the age of nineteen, he published the Discurs von der Großmächtigen Philosophischen Universal-Artzney / von den Philosophis genannt Lapis Philosophorum Trismegistus (discourse about the almighty philosophical and universal medicine by the philosopher called Lapis Philosophorum Trismegistus) under the pseudonym ‘Solinus Salzthal of Regiomontus.’ It was published in Latin in 1659 as Discursus Solini Saltztal Regiomontani De potentissima philosophorum medicina universali, lapis philosophorum trismegistus dicta (translated by Johannes Jacobus Heilmann) in vol. VI of the Theatrum Chemicum.
Career
In 1657, he was appointed professor of medicine at the University of Mainz and physician to the archbishop-elector. His Metallurgia was published in 1660; and the next year appeared his Character pro notitia linguarum universali, in which he gives 10,000 words for use as a universal language. In 1663, he published his Oedipum Chemicum and a book on animals, plants and minerals (Thier- Kräuter- und Bergbuch).
In 1666, he was made councillor of commerce (German: Commerzienrat) at Vienna, where he had gained the powerful support of the prime minister of Emperor Leopold I. Sent by the emperor on a mission to the Netherlands, he wrote there in ten days his Methodus Didactica, which was followed by the Regeln der Christlichen Bundesgenossenschaft and the Politischer Discurs von den eigentlichen Ursachen des Auf- und Abblühens der Städte, Länder und Republiken. In 1669, he published his Physica subterranea; the same year, he was engaged with the count of Hanau in a scheme to acquire Dutch colonization of Guiana from the Dutch West India Company.
Meanwhile, he had been appointed physician to the elector of Bavaria; but in 1670 he was again in Vienna advising on the establishment of a silk factory and propounding schemes for a great company to trade with the Low Countries and for a canal to unite the Rhine and Danube.
In 1678, he crossed to England. He travelled to Scotland where he visited the mines at the request of Prince Rupert. He afterwards travelled for the same purpose to Cornwall, and spent a year there. At the beginning of 1680, he presented a paper to the Royal Society in which he attempted to deprive Christiaan Huygens of the honour of applying the pendulum to the measurement of time. In 1682, he returned to London, where he wrote Närrische Weisheit und weise Narrheit (in which, according to Otto Mayr he made extensive references to temperature regulated furnaces), a book the Chymischer Glücks-Hafen, Oder Grosse Chymische Concordantz Und Collection, Von funffzehen hundert Chymischen Processen and died in October of the same year.
Legacy
Austrian Cameralist
Becher was the most original and influential theorist of Austrian cameralism. He sought to balance between the need to reinstate postwar levels of population and production both in the countryside and the towns. By leaning more seriously on trade and commerce, Austrian cameralism helped to transfer attention to the troubles of the monarchy’s urban economies. Ferdinand II had already taken some corrective steps before he died by attempting to ease the debts of the Bohemian towns and to put limits on some of the land-holding nobility’s commercial rights. Even though preceding Habsburgs had held the guilds responsible for their restrictiveness, wastefulness, and the poor value of the merchandise they created, Ferdinand II ramped up the pressure by extending rights to private artisans who usually then earned the fortification of powerful local leaders such as seigneurs, military commanders, churches, and universities. An edict by Leopold I in 1689 had granted the government the right to monitor and control the number of masters and cut down on the monopoly effect of guild operations. Even previous to this, Becher, who was against all forms of monopoly, surmised that a third of the Austrian lands’ 150,000 artisans were “Schwarzarbeiter” who were not in a guild.
Immediately after the Thirty Years’ War the Bohemian towns had petitioned Ferdinand to refine its own raw materials into more finished goods for export. Becher became the leading force in attempting this conversion. By 1666 he had inspired the creation of a Commerce Commission (Kommerzkollegium) in Vienna, as well as the reestablishment of the first postwar silk plantation on the Lower Austrian estates of Hofkammer President Sinzendorf. Becher then subsequently helped create a Kunst- und Werkhaus in which foreign masters trained non-guild artisans in the production of finished goods. By 1672 he had promoted the construction of a wool factory in Linz. Four years later he established a textile workhouse for vagabonds in the Boemian town of Tabor that eventually employed 186 spinners under his own directorship.
Some of Becher’s projects met with limited success. In time Linz’s new wool factory even became one of the largest and most important in Europe. Yet most of the government initiatives ended in failure. The Commerce Commission was doomed by Sinzendorf’s corruption and indifference. The Tabor workhouse nearly collapsed after just five years owing to the lack of government funding, and was then destroyed two years later during the Turkish invasion. The Oriental Company was fatally handicapped by a combination of poor management, government export prohibitions against Turkey, the opposition of Ottoman (principally Greek) merchants, and ultimately by the outbreak of war. The Kunst- und Werkhaus also folded during the 1680s, partly because of the regime’s unwillingness to import a significant number of foreign, Protestant teachers and skilled workers.
Chemist and alchemist
William Cullen considered Becher as a chemist of first importance and Physica Subterranea as the most considerable of Bechers writings.
Bill Bryson, in his A Short History of Nearly Everything, notes:
Chemistry as an earnest and respectable science is often said to date from 1661, when Robert Boyle of Oxford published The Sceptical Chymist — the first work to distinguish between chemists and alchemists — but it was a slow and often erratic transition. Into the eighteenth century scholars could feel oddly comfortable in both camps — like the German Johann Becher, who produced sober and unexceptionable work on mineralogy called Physica Subterranea, but who also was certain that, given the right materials, he could make himself invisible.
References
- Chisholm 1911, p. 602.
- Smith, Pamela H. (2016). The Business of Alchemy: Science and Culture in the Holy Roman Empire. Princeton: Princeton University Press. ISBN 9780691173238, p. 40/41; see also: ‘The Emperor’s Mercantile Alchemist’ in: Greenberg, Arthur (2006) – From Alchemy to Chemistry in Picture and Story. Hoboken N.J. : John Wiley & Sons. ISBN 978 0 470 08523 3. p. 231f. Chisholm writes in the 11th. ed. of the Encyclopædia Britannica that Becher “published an edition of Salzthal’s Tractatus de lapide trismegisto.”
- Chisholm 1911, pp. 602–603.
- Mayr, Otto (1971). “Adam Smith and the Concept of the Feedback System: Economic Thought and Technology in 18th-Century Britain”. Technology and Culture. 12 (1): 1–22. doi:10.2307/3102276. ISSN 0040-165X. JSTOR 3102276.
- Chisholm 1911, p. 603.
- Charles W. Ingrao, The Habsburg Monarchy: 1618-1815, New York: Cambridge University Press, Second edition. ISBN 0-521-78505-7; p. 92-93.
- Bill Bryson, A Short History of Nearly Everything, London: Black Swan, 2003 edition. ISBN 0-552-99704-8; p. 130.
Sources
This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). “Becher, Johann Joachim”. Encyclopædia Britannica. Vol. 3 (11th ed.). Cambridge University Press. pp. 602–603.
- Smith, Pamela H. (1994). The Business of Alchemy: Science and Culture in the Holy Roman Empire. Princeton: Princeton University Press.
- Ingrao, Charles W. (2005). The Habsburg Monarchy: 1618–1815. New York: Cambridge University Press.
Further reading
- Anthony Endres, Neoclassical Microeconomic Theory: the founding Austrian version (London: Routledge Press, 1997).
- Erik Grimmer-Solem, The Rise of Historical Economics and Social Reform in Germany 1864–1894 (New York: Oxford University Press, 2003).

In 1703, Georg Ernst Stahl, a professor of medicine and chemistry at Halle, proposed a variant of the theory in which he renamed Becher’s terra pinguis to phlogiston, and it was in this form that the theory probably had its greatest influence. The term ‘phlogiston’ itself was not something that Stahl invented. There is evidence that the word was used as early as 1606, and in a way that was very similar to what Stahl was using it for. The term was derived from a Greek word meaning inflame. The following paragraph describes Stahl’s view of phlogiston:
To Stahl, metals were compounds containing phlogiston in combination with metallic oxides (calces); when ignited, the phlogiston was freed from the metal leaving the oxide behind. When the oxide was heated with a substance rich in phlogiston, such as charcoal, the calx again took up phlogiston and regenerated the metal. Phlogiston was a definite substance, the same in all its combinations.[Leicester, Henry M. Klickstein, Herbert S. (1965). A Source Book in Chemistry. Cambridge, Massachusetts: Harvard University Press]
Stahl’s first definition of phlogiston first appeared in his Zymotechnia fundamentalis, published in 1697. His most quoted definition was found in the treatise on chemistry entitled Fundamenta chymiae in 1723. According to Stahl, phlogiston was a substance that was not able to be put into a bottle but could be transferred nonetheless. To him, wood was just a combination of ash and phlogiston, and making a metal was as simple as getting a metal calx and adding phlogiston. Soot was almost pure phlogiston, which is why heating it with a metallic calx transforms the calx into the metal and Stahl attempted to prove that the phlogiston in soot and sulphur were identical by converting sulphates to liver of sulphur using charcoal. He did not account for the increase in weight on combustion of tin and lead that were known at the time.
- White, John Henry (1973). The History of Phlogiston Theory. New York: AMS Press Inc. ISBN 978-0404069308.
- Leicester, Henry M.; Klickstein, Herbert S. (1965). A Source Book in Chemistry. Cambridge, Massachusetts: Harvard University Press.
- Mason, Stephen F., (1962). A History of the Sciences (revised edition). New York: Collier Books. Ch. 26.
- Ladenburg 1911, pp. 6–7.
- Wikipedia https://en.wikipedia.org/wiki/Phlogiston_theory
Georg Ernst Stahl (22 October 1659 – 24 May 1734) was a German chemist, physician and philosopher. He was a supporter of vitalism, and until the late 18th century his works on phlogiston were accepted as an explanation for chemical processes.
BIOGRAPHY
Georg Ernst Stahl was born on October 22, 1659, at Anspach in Bavaria. Raised as a son to a Lutheran Pastor, he was brought up in a very pious and religious household. From an early age he expressed profound interest toward chemistry, even by age 15 mastering a set of university lecture notes on chemistry and eventually a difficult treatise by Johann Kunckel. He had two wives, who both died from puerperal fever in 1696 and 1706. He also had a son Johnathan and a daughter who died in 1708. He continued to work and publish following the death of both of his wives and eventually his children, but was often very cold to students and fell into deep depression until his death in 1734 at the age of 74.
Life and education
He was born in St. John’s parish in Ansbach, Brandenburg on October 21, 1659. His father was Johann Lorentz Stahl. He was raised in Pietism, which influenced his viewpoints on the world. His interests in chemistry were due to the influence a professor of medicine, Jacob Barner, and a chemist, Johann Kunckel von Löwenstjern. In the late 1670s, Stahl moved to Saxe-Jena to study medicine at the University of Jena. Stahl’s success at Jena earned him a M.D. around 1683 and then he went on to teach at the same university.
Teaching at the university gained him such a good reputation that in 1687 he was hired as the personal physician to Duke Johann Ernst of Sachsen-Weimar. In 1693, he joined his old college friend Friedrich Hoffmann at the University of Halle. In 1694, he held the chair of medicine at the University of Halle. From 1715 until his death, he was the physician and counselor to King Friedrich Wilhelm I of Prussia and in charge of Berlin’s Medical Board.
Medicine
Stahl’s focus was on the distinction between the living and nonliving. Although he did not support the views of iatro-mechanists, he believed that all non-living creatures are mechanical and so are living things to a certain degree. His views were that nonliving things are stable throughout time and did not rapidly change. On the other hand, living things are subject to change and have a tendency to decompose, which led Stahl to work with fermentation.
Stahl professed an animistic system, in opposition to the materialism of Hermann Boerhaave and Friedrich Hoffmann. His main argument on living things was that there is an agent responsible for delaying this decomposition of living things and that agent is the anima or soul of the living organism. The anima controls all of the physical processes that happen in the body. It not only just controls the mechanical aspects of it but the direction and goals of them too. How the anima controls these processes is through motion. He believed that the three important motions of the body are the circulation of blood, excretion and secretion.
These beliefs were reflected in his views on medicine. He thought that medicine should deal with the body as a whole and its anima, rather than the specific parts of a body. Having knowledge on the specific mechanical parts of the body is not very useful. His views had been criticized by Gottfried Leibniz, with whom he exchanged letters, later published in a book titled Negotium otiosum seu σκιαμαχία (1720). Also, during the first part of the 18th century, Stahl’s ideas on the non-physical part of the body were disregarded while his mechanistic ideas on the body were accepted in the works of Boerhaave and Hoffmann.
Tonic motion
As a physician, Stahl worked with patients and focused on the soul, or anima, as well as blood circulation and tonic motion. Anima was a vital force that when working properly would allow the subject to be healthy; however, when malfunction of the anima occurred, so did illness. Tonic motion, to Stahl, involved the contracting and relaxing movements of the body tissue in order to serve the three main purposes. Tonic motion helped explain how animals produce heat and how fevers were caused. In Stahl’s 1692 dissertation, De motu tonico vitali, Stahl explains his theory of tonic motion and how it is connected to blood flow within a subject, without citing William Harvey’s blood flow and circulation theories, which lacked an explanation of irregular blood flow. Also within the dissertation, ‘practitioners’ are mentioned as users of his theory of tonic motion.
Stahl’s theory of tonic motion was about the muscle tone of the circulatory system. During his work at Halle, Stahl oversaw patients experiencing headaches and nosebleeds. Tonic motion explained these phenomena as blood needed a natural or artificial path to flow when a part of the body is obstructed, injured, or swollen. Stahl also experimented with menstruation, finding that bloodletting in an upper portion of the body would relieve bleeding during the period. During the next period, the wound would experience pain and swelling, which would only be relieved by an opening in the foot. He also followed this procedure as a treatment for amenorrhoea.
Chemistry
The best of Stahl’s work in chemistry was done while he was a professor at Halle. Just like medicine, he believed that chemistry could not be reduced to mechanistic views. Although he believed in atoms, he did not believe that atomic theories were enough to describe the chemical processes that go on. He believed that atoms could not be isolated individually and that they join to form elements. He took an empirical approach when establishing his descriptions of chemistry.
Stahl used the works of Johann Joachim Becher to help him come up with explanations of chemical phenomena. The main theory that Stahl got from J. J. Becher was the theory of phlogiston. This theory did not have any experimental basis before Stahl. Becher’s theories attempted in explaining chemistry as comprehensively as seemingly possible through classifying different earths according to specific reactions. Terra pinguis was a substance that escaped during combustion reactions, according to Becher. Stahl, influenced by Becher’s work, developed his theory of phlogiston. Phlogiston theory did not have any experimental basis before Stahl worked with metals and various other substances in order separate phlogiston from them. Stahl proposed that metals were made of calx, or ash, and phlogiston and that once a metal is heated, the phlogiston leaves only the calx within the substance. He was able to make the theory applicable to chemistry as it was one of the first unifying theories in the discipline. Phlogiston provided an explanation of various chemical phenomena and encouraged the chemists of the time to rationally work with the theory to explore more of the subject. This theory was later replaced by Antoine-Laurent Lavoisier’s theory of oxidation and caloric theory. He also propounded a view of fermentation, which in some respects resembles that supported by Justus von Liebig a century and half later. Although his theory was replaced, Stahl’s theory of phlogiston is seen to be the transition between alchemy and chemistry.
Stahl is credited for being among the first to describe carbon monoxide as noxious carbonarii halitus (carbonic vapors) in his 1697 publication Zymotechnia fundamentalis.
WORKS
- Zymotechnia fundamentalis (1697)
- De lumbricis terrestribus eorumque usu medico (in Latin). Halle: Christian Henckel. 1698.
- Disquisitio de mechanismi et organismi diversitate (1706)
- Paraenesis, ad aliena a medica doctrine arcendum (1706)
- De vera diversitate corporis mixti et vivi (1706)
- Theoria medica vera (1708)
- De lapide manati (in Latin). Halle: Christian Henckel. 1710.
- Georgii Ernesti Stahlii opusculum chymico-physico-medicum : seu schediasmatum, a pluribus annis variis occasionibus in publicum emissorum nunc quadantenus etiam auctorum et deficientibus passim exemplaribus in unum volumen iam collectorum, fasciculus publicae luci redditus / Praemißa praefationis loco authoris epistola ad Michaelem Alberti (1715) Digital edition by the University and State Library Düsseldorf
- Specimen Beccherianum (1718)
- Philosophical Principles of Universal Chemistry (1730), Peter Shaw, translator, from Open Library.
- Experimenta, observationes, animadversiones, 300. numero, chymicae et physicae (in Latin). Berlin: Ambrosius Haude. 1731.
- Materia medica : das ist: Zubereitung, Krafft und Würckung, derer sonderlich durch chymische Kunst erfundenen Artzneyen (1744), Vol. 1&2 Digital edition by the University and State Library Düsseldorf
- Fundamenta chymiae (in Latin). Vol. 3. Nürnberg: Wolfgang Moritz Endter, Erben & Julius Arnold Engelbrecht, Witwe. 1747.
- Fundamenta chymiae (in Latin). Vol. 2. Nürnberg: Wolfgang Moritz Endter, Erben & Julius Arnold Engelbrecht, Witwe. 1746.
- The Leibniz-Stahl Controversy (2016), transl. and edited by F. Duchesneau and J. H. Smith, Yale UP (536 pp.)
References
- Stahl’s date of birth is often given erroneously as 1660. The correct date is recorded in the parish register of St. John’s church, Ansbach. See Gottlieb, B.J. (1942). “Vitalistisches Denken in Deutschland im Anschluss an Georg Ernst Stahl”. Klinische Wochenschrift. 21 (20): 445–448. doi:10.1007/bf01773817. S2CID 41987182.
- Ku-ming Chang (2008)”Stahl, Georg Ernst”, Complete Dictionary of Scientific Biography, Vol. 24, from Cengage Learning
- “Adreßbuch Deutscher Chemiker 1953/54. Gemeinsam herausgegeben von Gesellschaft Deutscher Chemiker und Verlag Chemie, Weinheim/Bergstraße. Verlag Chemie GmbH. (1953). 450 S.”. Starch – Stärke. 6 (12): 312. 1954. doi:10.1002/star.19540061211. ISSN 0038-9056.
- “Georg Ernst Stahl”. Encyclopædia Britannica, Encyclopædia Britannica Online. Encyclopædia Britannica Inc., 2013. Web. 25 May 2013
- Magill, Frank N. “Georg Ernst Stahl”, Dictionary of World Biography. 1st ed. 1999. Print.
- Francesco Paolo de Ceglia: Hoffmann and Stahl. Documents and Reflections on the Dispute. in History of Universities 22/1 (2007): 98–140.
- Smets, Alexis. The Controversy Between Leibniz and Stahl on the Theory of Chemistry Archived 2012-04-25 at the Wayback Machine, Proceedings of the 6th International Conference on History of Chemistry
- The Leibniz-Stahl Controversy (2016), transl. and edited by F. Duchesneau and J.H. Smith, Yale UP (536pp.)
- Vartanian, Aram (2006) “Stahl, Georg Ernst (1660–1734)”, Encyclopedia of Philosophy, editor Donald M. Borchert. 2nd ed. Vol. 9. Detroit: Macmillan Reference USA. 202–203. Gale Virtual Reference Library. Web. 26 May 2013.
- Chang, K (2004). “Motus Tonicus: Georg Ernst Stahl’s Formulation of Tonic Motion and Early Modern Medical Thought”. Bulletin of the History of Medicine. 78 (4): 767–803. doi:10.1353/bhm.2004.0161. PMID 15591695. S2CID 12488842. Retrieved 20 April 2016.
- Hudson, John (1992). The History of Chemistry. Hong Kong: The Macmillan Press. p. 47. ISBN 0-412-03641-X.
- Hopper, Christopher P.; Zambrana, Paige N.; Goebel, Ulrich; Wollborn, Jakob (June 2021). “A brief history of carbon monoxide and its therapeutic origins”. Nitric Oxide. 111–112: 45–63. doi:10.1016/j.niox.2021.04.001.
- Hélène Metzger (1926) “La philosophie de la matière chez Stahl et ses disciples”, Isis 8: 427–464.
- Hélène Metzger (1930) Newton, Stahl, Boerhaave et la Doctrine Chemique
- Lawrence M. Principe (2007) Chymists and Chymistry.
- Wikipedia https://en.wikipedia.org/wiki/Georg_Ernst_Stahl

Phlogiston remained the dominant theory until the 1770s when Antoine-Laurent de Lavoisier showed that combustion requires a gas that has weight (specifically, oxygen) and could be measured by means of weighing closed vessels. The use of closed vessels by Lavoisier and earlier by the Russian scientist Mikhail Lomonosov also negated the buoyancy that had disguised the weight of the gases of combustion, and culminated in the principle of mass conservation. These observations solved the mass paradox and set the stage for the new oxygen theory of combustion.
Dismantling phlogiston theory
Lavoisier’s chemical research between 1772 and 1778 was largely concerned with developing his own new theory of combustion. In 1783 he read to the academy his paper entitled Réflexions sur le phlogistique (Reflections on Phlogiston), a full-scale attack on the current phlogiston theory of combustion. That year Lavoisier also began a series of experiments on the composition of water which were to prove an important capstone to his combustion theory and win many converts to it. Many investigators had been experimenting with the combination of Henry Cavendish’s inflammable air, now known as hydrogen, with “dephlogisticated air” (air in the process of combustion, now known to be oxygen) by electrically sparking mixtures of the gases. All of the researchers noted Cavendish’s production of pure water by burning hydrogen in oxygen, but they interpreted the reaction in varying ways within the framework of phlogiston theory. Lavoisier learned of Cavendish’s experiment in June 1783 via Charles Blagden (before the results were published in 1784), and immediately recognized water as the oxide of a hydroelectric gas.
In cooperation with Laplace, Lavoisier synthesized water by burning jets of hydrogen and oxygen in a bell jar over mercury. The quantitative results were good enough to support the contention that water was not an element, as had been thought for over 2,000 years, but a compound of two gases, hydrogen and oxygen. The interpretation of water as a compound explained the inflammable air generated from dissolving metals in acids (hydrogen produced when water decomposes) and the reduction of calces by inflammable air (a combination of gas from calx with oxygen to form water).
Despite these experiments, Lavoisier’s antiphlogistic approach remained unaccepted by many other chemists. Lavoisier labored to provide definitive proof of the composition of water, attempting to use this in support of his theory. Working with Jean-Baptiste Meusnier, Lavoisier passed water through a red-hot iron gun barrel, allowing the oxygen to form an oxide with the iron and the hydrogen to emerge from the end of the pipe. He submitted his findings of the composition of water to the Académie des Sciences in April 1784, reporting his figures to eight decimal places. Opposition responded to this further experimentation by stating that Lavoisier continued to draw the incorrect conclusions and that his experiment demonstrated the displacement of phlogiston from iron by the combination of water with the metal. Lavoisier developed a new apparatus which used a pneumatic trough, a set of balances, a thermometer, and a barometer, all calibrated carefully. Thirty savants were invited to witness the decomposition and synthesis of water using this apparatus, convincing many who attended of the correctness of Lavoisier’s theories. This demonstration established water as a compound of oxygen and hydrogen with great certainty for those who viewed it. The dissemination of the experiment, however, proved subpar, as it lacked the details to properly display the amount of precision taken in the measurements. The paper ended with a hasty statement that the experiment was “more than sufficient to lay hold of the certainty of the proposition” of the composition of water and stated that the methods used in the experiment would unite chemistry with the other physical sciences and advance discoveries.
- Best, Nicholas W. (1 July 2015). “Lavoisier’s “Reflections on phlogiston” I: against phlogiston theory”. Foundations of Chemistry. 17 (2): 137–151. doi:10.1007/s10698-015-9220-5. ISSN 1572-8463. S2CID 254510272.
- Ihde, Aaron (1964). The Development of Modern Chemistry. New York: Harper & Row. p. 81.
- Gillispie, Charles Coulston (1960). The Edge of Objectivity: An Essay in the History of Scientific Ideas. Princeton University Press. p. 228. ISBN 0-691-02350-6.
- Lavoisier and Meusnier, “Développement” (cit. n. 27), pp. 205–209; cf. Holmes, Lavoisier (cn. 8), p. 237.
- Golinski, Jan (1994). “Precision instruments and the demonstrative order of proof in Lavoisier’s chemistry” (PDF). Osiris. 9: 30–47. doi:10.1086/368728. JSTOR 301997. S2CID 95978870
- Wikipedia https://en.wikipedia.org/wiki/Phlogiston_theory
- Wikipedia https://en.wikipedia.org/wiki/Antoine_Lavoisier
Antoine-Laurent de Lavoisier (26 August 1743 – 8 May 1794), also Antoine Lavoisier after the French Revolution, was a French nobleman and chemist who was central to the 18th-century chemical revolution and who had a large influence on both the history of chemistry and the history of biology.
It is generally accepted that Lavoisier’s great accomplishments in chemistry stem largely from his changing the science from a qualitative to a quantitative one. Lavoisier is most noted for his discovery of the role oxygen plays in combustion. He named oxygen (1778), recognizing it as an element, and also recognized hydrogen as an element (1783), opposing the phlogiston theory.
Lavoisier helped construct the metric system, wrote the first extensive list of elements, and helped to reform chemical nomenclature. He predicted the existence of silicon (1787) and discovered that, although matter may change its form or shape, its mass always remains the same. His wife and laboratory assistant, Marie-Anne Paulze Lavoisier, became a renowned chemist in her own right.
Lavoisier was a powerful member of a number of aristocratic councils, and an administrator of the Ferme générale. The Ferme générale was one of the most hated components of the Ancien Régime because of the profits it took at the expense of the state, the secrecy of the terms of its contracts, and the violence of its armed agents. All of these political and economic activities enabled him to fund his scientific research. At the height of the French Revolution, he was charged with tax fraud and selling adulterated tobacco, and was guillotined.
Biography
Early life and education
Antoine-Laurent Lavoisier was born to a wealthy family of the nobility in Paris on 26 August 1743. The son of an attorney at the Parlement of Paris, he inherited a large fortune at the age of five upon the death of his mother. Lavoisier began his schooling at the Collège des Quatre-Nations, University of Paris (also known as the Collège Mazarin) in Paris in 1754 at the age of 11. In his last two years (1760–1761) at the school, his scientific interests were aroused, and he studied chemistry, botany, astronomy, and mathematics. In the philosophy class he came under the tutelage of Abbé Nicolas Louis de Lacaille, a distinguished mathematician and observational astronomer who imbued the young Lavoisier with an interest in meteorological observation, an enthusiasm which never left him. Lavoisier entered the school of law, where he received a bachelor’s degree in 1763 and a licentiate in 1764. Lavoisier received a law degree and was admitted to the bar, but never practiced as a lawyer. However, he continued his scientific education in his spare time.
Early scientific work
Lavoisier’s education was filled with the ideals of the French Enlightenment of the time, and he was fascinated by Pierre Macquer’s dictionary of chemistry. He attended lectures in the natural sciences. Lavoisier’s devotion and passion for chemistry were largely influenced by Étienne Condillac, a prominent French scholar of the 18th century. His first chemical publication appeared in 1764. From 1763 to 1767, he studied geology under Jean-Étienne Guettard. In collaboration with Guettard, Lavoisier worked on a geological survey of Alsace-Lorraine in June 1767. In 1764 he read his first paper to the French Academy of Sciences, France’s most elite scientific society, on the chemical and physical properties of gypsum (hydrated calcium sulfate), and in 1766 he was awarded a gold medal by the King for an essay on the problems of urban street lighting. In 1768 Lavoisier received a provisional appointment to the Academy of Sciences. In 1769, he worked on the first geological map of France.
Lavoisier as a social reformer
Research benefitting the public good
While Lavoisier is commonly known for his contributions to the sciences, he also dedicated a significant portion of his fortune and work toward benefitting the public. Lavoisier was a humanitarian—he cared deeply about the people in his country and often concerned himself with improving the livelihood of the population by agriculture, industry, and the sciences. The first instance of this occurred in 1765, when he submitted an essay on improving urban street lighting to the French Academy of Sciences.
Three years later in 1768, he focused on a new project to design an aqueduct. The goal was to bring water from the river Yvette into Paris so that the citizens could have clean drinking water. But, since the construction never commenced, he instead turned his focus to purifying the water from the Seine. This was the project that interested Lavoisier in the chemistry of water and public sanitation duties.
Additionally, he was interested in air quality and spent some time studying the health risks associated with gunpowder’s effect on the air. In 1772, he performed a study on how to reconstruct the Hôtel-Dieu hospital, after it had been damaged by fire, in a way that would allow proper ventilation and clean air throughout.
At the time, the prisons in Paris were known to be largely unlivable and the prisoners’ treatment inhumane. Lavoisier took part in investigations in 1780 (and again in 1791) on the hygiene in prisons and had made suggestions to improve living conditions, suggestions which were largely ignored.
Once a part of the Academy, Lavoisier also held his own competitions to push the direction of research towards bettering the public and his own work.
Sponsorship of the sciences
Lavoisier had a vision of public education having roots in “scientific sociability” and philanthropy.
Lavoisier gained a vast majority of his income through buying stock in the General Farm, which allowed him to work on science full-time, live comfortably, and allowed him to contribute financially to better the community. (It would also contribute to his demise during the Reign of Terror many years later.)
It was very difficult to secure public funding for the sciences at the time, and additionally not very financially profitable for the average scientist, so Lavoisier used his wealth to open a very expensive and sophisticated laboratory in France so that aspiring scientists could study without the barriers of securing funding for their research.
He also pushed for public education in the sciences. He founded two organizations, Lycée [fr] and Musée des Arts et Métiers, which were created to serve as educational tools for the public. Funded by the wealthy and noble, the Lycée regularly taught courses to the public beginning in 1793.
Ferme générale and marriage
At the age of 26, around the time he was elected to the Academy of Sciences, Lavoisier bought a share in the Ferme générale, a tax farming financial company which advanced the estimated tax revenue to the royal government in return for the right to collect the taxes. On behalf of the Ferme générale Lavoisier commissioned the building of a wall around Paris so that customs duties could be collected from those transporting goods into and out of the city. His participation in the collection of its taxes did not help his reputation when the Reign of Terror began in France, as taxes and poor government reform were the primary motivators during the French Revolution.
Lavoisier consolidated his social and economic position when, in 1771 at age 28, he married Marie-Anne Pierrette Paulze, the 13-year-old daughter of a senior member of the Ferme générale. She was to play an important part in Lavoisier’s scientific career—notably, she translated English documents for him, including Richard Kirwan’s Essay on Phlogiston and Joseph Priestley’s research. In addition, she assisted him in the laboratory and created many sketches and carved engravings of the laboratory instruments used by Lavoisier and his colleagues for their scientific works. Madame Lavoisier edited and published Antoine’s memoirs (whether any English translations of those memoirs have survived is unknown as of today) and hosted parties at which eminent scientists discussed ideas and problems related to chemistry.
A portrait of Antoine and Marie-Anne Lavoisier was painted by the famed artist Jacques-Louis David. Completed in 1788 on the eve of the Revolution, the painting was denied a customary public display at the Paris Salon for fear that it might inflame anti-aristocratic passions.
For three years following his entry into the Ferme générale, Lavoisier’s scientific activity diminished somewhat, for much of his time was taken up with official Ferme générale business. He did, however, present one important memoir to the Academy of Sciences during this period, on the supposed conversion of water into earth by evaporation. By a very precise quantitative experiment, Lavoisier showed that the “earthy” sediment produced after long-continued reflux heating of water in a glass vessel was not due to a conversion of the water into earth but rather to the gradual disintegration of the inside of the glass vessel produced by the boiling water. He also attempted to introduce reforms in the French monetary and taxation system to help the peasants.
Adulteration of tobacco
The Farmers General held a monopoly of the production, import and sale of tobacco in France, and the taxes they levied on tobacco brought revenues of 30 million livres a year. This revenue began to fall because of a growing black market in tobacco that was smuggled and adulterated, most commonly with ash and water. Lavoisier devised a method of checking whether ash had been mixed in with tobacco: “When a spirit of vitriol, aqua fortis or some other acid solution is poured on ash, there is an immediate very intense effervescent reaction, accompanied by an easily detected noise.” Lavoisier also noticed that the addition of a small amount of ash improved the flavour of tobacco. Of one vendor selling adulterated goods, he wrote “His tobacco enjoys a very good reputation in the province… the very small proportion of ash that is added gives it a particularly pungent flavour that consumers look for. Perhaps the Farm could gain some advantage by adding a bit of this liquid mixture when the tobacco is fabricated.” Lavoisier also found that while adding a lot of water to bulk the tobacco up would cause it to ferment and smell bad, the addition of a very small amount improved the product. Thereafter the factories of the Farmers General added, as he recommended, a consistent 6.3% of water by volume to the tobacco they processed. To allow for this addition, the Farmers General delivered to retailers seventeen ounces of tobacco while only charging for sixteen. To ensure that only these authorised amounts were added, and to exclude the black market, Lavoisier saw to it that a watertight system of checks, accounts, supervision and testing made it very difficult for retailers to source contraband tobacco or to improve their profits by bulking it up. He was energetic and rigorous in implementing this, and the systems he introduced were deeply unpopular with the tobacco retailers across the country. This unpopularity was to have consequences for him during the French Revolution.
Royal Commission on Agriculture
Lavoisier urged the establishment of a Royal Commission on Agriculture. He then served as its Secretary and spent considerable sums of his own money in order to improve the agricultural yields in the Sologne, an area where farmland was of poor quality. The humidity of the region often led to a blight of the rye harvest, causing outbreaks of ergotism among the population. In 1788 Lavoisier presented a report to the Commission detailing ten years of efforts on his experimental farm to introduce new crops and types of livestock. His conclusion was that despite the possibilities of agricultural reforms, the tax system left tenant farmers with so little that it was unrealistic to expect them to change their traditional practices.
Gunpowder Commission
Lavoisier’s researches on combustion were carried out in the midst of a very busy schedule of public and private duties, especially in connection with the Ferme Générale. There were also innumerable reports for and committees of the Academy of Sciences to investigate specific problems on order of the royal government. Lavoisier, whose organizing skills were outstanding, frequently landed the task of writing up such official reports. In 1775 he was made one of four commissioners of gunpowder appointed to replace a private company, similar to the Ferme Générale, which had proved unsatisfactory in supplying France with its munitions requirements. As a result of his efforts, both the quantity and quality of French gunpowder greatly improved, and it became a source of revenue for the government. His appointment to the Gunpowder Commission brought one great benefit to Lavoisier’s scientific career as well. As a commissioner, he enjoyed both a house and a laboratory in the Royal Arsenal. Here he lived and worked between 1775 and 1792.
Lavoisier was a formative influence in the formation of the Du Pont gunpowder business because he trained Éleuthère Irénée du Pont, its founder, on gunpowder-making in France; the latter said that the Du Pont gunpowder mills “would never have been started but for his kindness to me.”
During the Revolution
In June 1791, Lavoisier made a loan of 71,000 livres to Pierre Samuel du Pont de Nemours to buy a printing works so that du Pont could publish a newspaper, La Correspondance Patriotique. The plan was for this to include both reports of debates in the National Constituent Assembly as well as papers from the Academy of Sciences. The revolution quickly disrupted the elder du Pont’s first newspaper, but his son E.I. du Pont soon launched Le Republicain and published Lavoisier’s latest chemistry texts.
Lavoisier also chaired the commission set up to establish a uniform system of weights and measures which in March 1791 recommended the adoption of the metric system. The new system of weights and measures was adopted by the Convention on 1 August 1793. Lavoisier was one of the 27 Farmers General who, by order of the Convention, were all to be detained. Although temporarily going into hiding, on 30 November 1793 he handed himself into the Port Royal convent for questioning. He claimed he had not operated on this commission for many years, having instead devoted himself to science.
Lavoisier himself was removed from the commission on weights and measures on 23 December 1793, together with mathematician Pierre-Simon Laplace and several other members, for political reasons.
One of his last major works was a proposal to the National Convention for the reform of French education. He also intervened on behalf of a number of foreign-born scientists including mathematician Joseph Louis Lagrange, helping to exempt them from a mandate stripping all foreigners of possessions and freedom.
Final days and execution
As the French Revolution gained momentum, attacks mounted on the deeply unpopular Ferme générale, and it was eventually abolished in March 1791. In 1792 Lavoisier was forced to resign from his post on the Gunpowder Commission and to move from his house and laboratory at the Royal Arsenal. On 8 August 1793, all the learned societies, including the Academy of Sciences, were suppressed at the request of Abbé Grégoire.
On 24 November 1793, the arrest of all the former tax farmers was ordered. Lavoisier and the other Farmers General faced nine accusations of defrauding the state of money owed to it, and of adding water to tobacco before selling it. Lavoisier drafted their defense, refuting the financial accusations, reminding the court of how they had maintained a consistently high quality of tobacco. The court, however, was inclined to believe that by condemning them and seizing the goods of the Farmers General, it would recover huge sums for the state. Lavoisier was convicted and guillotined on 8 May 1794 in Paris, at the age of 50, along with his 27 co-defendants.
According to popular legend, the appeal to spare his life, in order that he could continue his experiments, was cut short by the judge, Coffinhal: “La République n’a pas besoin de savants ni de chimistes; le cours de la justice ne peut être suspendu.” (“The Republic needs neither scholars nor chemists; the course of justice cannot be delayed.”) The judge Coffinhal himself would be executed less than three months later, in the wake of the Thermidorian reaction.
Lavoisier’s importance to science was expressed by Lagrange who lamented the beheading by saying: “Il ne leur a fallu qu’un moment pour faire tomber cette tête, et cent années peut-être ne suffiront pas pour en reproduire une semblable.” (“It took them only an instant to cut off this head, and one hundred years might not suffice to reproduce its like.”)
Exoneration
A year and a half after his execution, Lavoisier was completely exonerated by the French government. During the White Terror, his belongings were delivered to his widow. A brief note was included, reading “To the widow of Lavoisier, who was falsely convicted”.
Contributions to chemistry
Oxygen theory of combustion
Contrary to prevailing thought at the time, Lavoisier theorized that common air, or one of its components, combines with substances when they are burned. He demonstrated this through experiment.
During late 1772 Lavoisier turned his attention to the phenomenon of combustion, the topic on which he was to make his most significant contribution to science. He reported the results of his first experiments on combustion in a note to the Academy on 20 October, in which he reported that when phosphorus burned, it combined with a large quantity of air to produce acid spirit of phosphorus, and that the phosphorus increased in weight on burning. In a second sealed note deposited with the Academy a few weeks later (1 November) Lavoisier extended his observations and conclusions to the burning of sulfur and went on to add that “what is observed in the combustion of sulfur and phosphorus may well take place in the case of all substances that gain in weight by combustion and calcination: and I am persuaded that the increase in weight of metallic calces is due to the same cause.”[citation needed]
Joseph Black’s “fixed air”
During 1773 Lavoisier determined to review thoroughly the literature on air, particularly “fixed air,” and to repeat many of the experiments of other workers in the field. He published an account of this review in 1774 in a book entitled Opuscules physiques et chimiques (Physical and Chemical Essays). In the course of this review, he made his first full study of the work of Joseph Black, the Scottish chemist who had carried out a series of classic quantitative experiments on the mild and caustic alkalies. Black had shown that the difference between a mild alkali, for example, Chalk (CaCO), and the caustic form, for example, quicklime (CaO), lay in the fact that the former contained “fixed air,” not common air fixed in the chalk, but a distinct chemical species, now understood to be carbon dioxide (CO2), which was a constituent of the atmosphere. Lavoisier recognized that Black’s fixed air was identical with the air evolved when metal calces were reduced with charcoal and even suggested that the air which combined with metals on calcination and increased the weight might be Black’s fixed air, that is, CO2.[citation needed]
Joseph Priestley
In the spring of 1774, Lavoisier carried out experiments on the calcination of tin and lead in sealed vessels, the results of which conclusively confirmed that the increase in weight of metals in combustion was due to combination with air. But the question remained about whether it was in combination with common atmospheric air or with only a part of atmospheric air. In October the English chemist Joseph Priestley visited Paris, where he met Lavoisier and told him of the air which he had produced by heating the red calx of mercury with a burning glass and which had supported combustion with extreme vigor. Priestley at this time was unsure of the nature of this gas, but he felt that it was an especially pure form of common air. Lavoisier carried out his own research on this peculiar substance. The result was his memoir On the Nature of the Principle Which Combines with Metals during Their Calcination and Increases Their Weight, read to the Academy on 26 April 1775 (commonly referred to as the Easter Memoir). In the original memoir, Lavoisier showed that the mercury calx was a true metallic calx in that it could be reduced with charcoal, giving off Black’s fixed air in the process. When reduced without charcoal, it gave off an air which supported respiration and combustion in an enhanced way. He concluded that this was just a pure form of common air and that it was the air itself “undivided, without alteration, without decomposition” which combined with metals on calcination.[citation needed]
After returning from Paris, Priestley took up once again his investigation of the air from mercury calx. His results now showed that this air was not just an especially pure form of common air but was “five or six times better than common air, for the purpose of respiration, inflammation, and … every other use of common air”. He called the air dephlogisticated air, as he thought it was common air deprived of its phlogiston. Since it was therefore in a state to absorb a much greater quantity of phlogiston given off by burning bodies and respiring animals, the greatly enhanced combustion of substances and the greater ease of breathing in this air were explained.[citation needed]
Pioneer of stoichiometry
Lavoisier’s researches included some of the first truly quantitative chemical experiments. He carefully weighed the reactants and products of a chemical reaction in a sealed glass vessel so that no gases could escape, which was a crucial step in the advancement of chemistry. In 1774, he showed that, although matter can change its state in a chemical reaction, the total mass of matter is the same at the end as at the beginning of every chemical change. Thus, for instance, if a piece of wood is burned to ashes, the total mass remains unchanged if gaseous reactants and products are included. Lavoisier’s experiments supported the law of conservation of mass. In France it is taught as Lavoisier’s Law and is paraphrased from a statement in his Traité Élémentaire de Chimie: “Nothing is lost, nothing is created, everything is transformed.” Mikhail Lomonosov (1711–1765) had previously expressed similar ideas in 1748 and proved them in experiments; others whose ideas pre-date the work of Lavoisier include Jean Rey (1583–1645), Joseph Black (1728–1799), and Henry Cavendish (1731–1810).
Chemical nomenclature
Lavoisier, together with Louis-Bernard Guyton de Morveau, Claude-Louis Berthollet, and Antoine François de Fourcroy, submitted a new program for the reforms of chemical nomenclature to the Academy in 1787, for there was virtually no rational system of chemical nomenclature at this time. This work, titled Méthode de nomenclature chimique (Method of Chemical Nomenclature, 1787), introduced a new system which was tied inextricably to Lavoisier’s new oxygen theory of chemistry.
The classical elements of earth, air, fire, and water were discarded, and instead some 33 substances which could not be decomposed into simpler substances by any known chemical means were provisionally listed as elements. The elements included light; caloric (matter of heat); the principles of oxygen, hydrogen, and azote (nitrogen); carbon; sulfur; phosphorus; the yet unknown “radicals” of muriatic acid (hydrochloric acid), boric acid, and “fluoric” acid; 17 metals; 5 earths (mainly oxides of yet unknown metals such as magnesia, baria, and strontia); three alkalies (potash, soda, and ammonia); and the “radicals” of 19 organic acids.
The acids, regarded in the new system as compounds of various elements with oxygen, were given names which indicated the element involved together with the degree of oxygenation of that element, for example sulfuric and sulfurous acids, phosphoric and phosphorous acids, nitric and nitrous acids, the “ic” termination indicating acids with a higher proportion of oxygen than those with the “ous” ending.
Similarly, salts of the “ic” acids were given the terminal letters “ate,” as in copper sulfate, whereas the salts of the “ous” acids terminated with the suffix “ite,” as in copper sulfite.
The total effect of the new nomenclature can be gauged by comparing the new name “copper sulfate” with the old term “vitriol of Venus.” Lavoisier’s new nomenclature spread throughout Europe and to the United States and became common use in the field of chemistry. This marked the beginning of the anti-phlogistic approach to the field.[citation needed]
Chemical revolution and opposition
Lavoisier is commonly cited as a central contributor to the chemical revolution. His precise measurements and meticulous keeping of balance sheets throughout his experiment were vital to the widespread acceptance of the law of conservation of mass. His introduction of new terminology, a binomial system modeled after that of Linnaeus, also helps to mark the dramatic changes in the field which are referred to generally as the chemical revolution. Lavoisier encountered much opposition in trying to change the field, especially from British phlogistic scientists. Joseph Priestley, Richard Kirwan, James Keir, and William Nicholson, among others, argued that quantification of substances did not imply conservation of mass. Rather than reporting factual evidence, opposition claimed Lavoisier was misinterpreting the implications of his research. One of Lavoisier’s allies, Jean Baptiste Biot, wrote of Lavoisier’s methodology, “one felt the necessity of linking accuracy in experiments to rigor of reasoning.” His opposition argued that precision in experimentation did not imply precision in inferences and reasoning. Despite opposition, Lavoisier continued to use precise instrumentation to convince other chemists of his conclusions, often results to five to eight decimal places. Nicholson, who estimated that only three of these decimal places were meaningful, stated:
If it be denied that these results are pretended to be true in the last figures, I must beg leave to observe, that these long rows of figures, which in some instances extend to a thousand times the nicety of experiment, serve only to exhibit a parade which true science has no need of: and, more than this, that when the real degree of accuracy in experiments is thus hidden from our contemplation, we are somewhat disposed to doubt whether the exactitude scrupuleuse of the experiments be indeed such as to render the proofs de l’ordre demonstratif.
Notable works
Easter memoir
The “official” version of Lavoisier’s Easter Memoir appeared in 1778. In the intervening period, Lavoisier had ample time to repeat some of Priestley’s latest experiments and perform some new ones of his own. In addition to studying Priestley’s dephlogisticated air, he studied more thoroughly the residual air after metals had been calcined. He showed that this residual air supported neither combustion nor respiration and that approximately five volumes of this air added to one volume of the dephlogisticated air gave common atmospheric air. Common air was then a mixture of two distinct chemical species with quite different properties. Thus when the revised version of the Easter Memoir was published in 1778, Lavoisier no longer stated that the principle which combined with metals on calcination was just common air but “nothing else than the healthiest and purest part of the air” or the “eminently respirable part of the air”. The same year he coined the name oxygen for this constituent of the air, from the Greek words meaning “acid former”. He was struck by the fact that the combustion products of such nonmetals as sulfur, phosphorus, charcoal, and nitrogen were acidic. He held that all acids contained oxygen and that oxygen was therefore the acidifying principle.
Dismantling phlogiston theory
already covered above
Elementary Treatise of Chemistry
Lavoisier employed the new nomenclature in his Traité élémentaire de chimie (Elementary Treatise on Chemistry), published in 1789. This work represents the synthesis of Lavoisier’s contribution to chemistry and can be considered the first modern textbook on the subject. The core of the work was the oxygen theory, and the work became a most effective vehicle for the transmission of the new doctrines. It presented a unified view of new theories of chemistry, contained a clear statement of the law of conservation of mass, and denied the existence of phlogiston. This text clarified the concept of an element as a substance that could not be broken down by any known method of chemical analysis and presented Lavoisier’s theory of the formation of chemical compounds from elements. It remains a classic in the history of science. While many leading chemists of the time refused to accept Lavoisier’s new ideas, demand for Traité élémentaire as a textbook in Edinburgh was sufficient to merit translation into English within about a year of its French publication. In any event, the Traité élémentaire was sufficiently sound to convince the next generation.
Physiological work
The relationship between combustion and respiration had long been recognized from the essential role which air played in both processes. Lavoisier was almost obliged, therefore, to extend his new theory of combustion to include the area of respiration physiology. His first memoirs on this topic were read to the Academy of Sciences in 1777, but his most significant contribution to this field was made in the winter of 1782–1783 in association with Laplace. The result of this work was published in a memoir, “On Heat.” Lavoisier and Laplace designed an ice calorimeter apparatus for measuring the amount of heat given off during combustion or respiration. The outer shell of the calorimeter was packed with snow, which melted to maintain a constant temperature of 0 °C around an inner shell filled with ice. By measuring the quantity of carbon dioxide and heat produced by confining a live guinea pig in this apparatus, and by comparing the amount of heat produced when sufficient carbon was burned in the ice calorimeter to produce the same amount of carbon dioxide as that which the guinea pig exhaled, they concluded that respiration was, in fact, a slow combustion process. Lavoisier stated, “la respiration est donc une combustion,” that is, respiratory gas exchange is a combustion, like that of a candle burning.
This continuous slow combustion, which they supposed took place in the lungs, enabled the living animal to maintain its body temperature above that of its surroundings, thus accounting for the puzzling phenomenon of animal heat. Lavoisier continued these respiration experiments in 1789–1790 in cooperation with Armand Seguin. They designed an ambitious set of experiments to study the whole process of body metabolism and respiration using Seguin as a human guinea pig in the experiments. Their work was only partially completed and published because of the Revolution’s disruption, but Lavoisier’s pioneering work in this field inspired similar research on physiological processes for generations.
Legacy
Lavoisier’s fundamental contributions to chemistry were a result of a conscious effort to fit all experiments into the framework of a single theory. He established the consistent use of the chemical balance, used oxygen to overthrow the phlogiston theory, and developed a new system of chemical nomenclature which held that oxygen was an essential constituent of all acids (which later turned out to be erroneous).
Lavoisier also did early research in physical chemistry and thermodynamics in joint experiments with Laplace. They used a calorimeter to estimate the heat evolved per unit of carbon dioxide produced, eventually finding the same ratio for a flame and animals, indicating that animals produced energy by a type of combustion reaction.
Lavoisier also contributed to early ideas on composition and chemical changes by stating the radical theory, believing that radicals, which function as a single group in a chemical process, combine with oxygen in reactions. He also introduced the possibility of allotropy in chemical elements when he discovered that diamond is a crystalline form of carbon.
He was also responsible for the construction of the gasometer, an expensive instrument he used at his demonstrations. While he used his gasometer exclusively for these, he also created smaller, cheaper, more practical gasometers that worked with a sufficient degree of precision that more chemists could recreate.
Overall, his contributions are considered the most important in advancing chemistry to the level reached in physics and mathematics during the 18th century.
Following his death, a collection comprising most of his scientific manuscripts and instruments was established by his relatives at the Château de la Canière in Puy-de-Dôme.
Mount Lavoisier in New Zealand’s Paparoa Range was named after him in 1970 by the Department of Scientific and Industrial Research.
Awards and honours
During his lifetime, Lavoisier was awarded a gold medal by the King of France for his work on urban street lighting (1766), and was appointed to the French Academy of Sciences (1768). He was elected as a member of the American Philosophical Society in 1775.
Lavoisier’s work was recognized as an International Historic Chemical Landmark by the American Chemical Society, Académie des sciences de L’institut de France and the Société Chimique de France in 1999. Antoine Laurent Lavoisier’s Louis 1788 publication entitled Méthode de Nomenclature Chimique, published with colleagues Louis-Bernard Guyton de Morveau, Claude Louis Berthollet, and Antoine François, comte de Fourcroy, was honored by a Citation for Chemical Breakthrough Award from the Division of History of Chemistry of the American Chemical Society, presented at the Académie des Sciences (Paris) in 2015.
The work of Lavoisier was translated in Japan in the 1840s, through the process of Rangaku. Page from Udagawa Yōan’s 1840 Seimi Kaisō
A number of Lavoisier Medals have been named and given in Lavoisier’s honour, by organizations including the Société chimique de France, the International Society for Biological Calorimetry, and the DuPont company. He is also commemorated by the Franklin-Lavoisier Prize, marking the friendship of Antoine-Laurent Lavoisier and Benjamin Franklin. The prize, which includes a medal, is given jointly by the Fondation de la Maison de la Chimie in Paris, France and the Science History Institute in Philadelphia, PA, USA.

Selected writings
- Opuscules physiques et chimiques (Paris: Chez Durand, Didot, Esprit, 1774). (Second edition, 1801)
- L’art de fabriquer le salin et la potasse, publié par ordre du Roi, par les régisseurs-généraux des Poudres & Salpêtres (Paris, 1779).
- Instruction sur les moyens de suppléer à la disette des fourrages, et d’augmenter la subsistence des bestiaux, Supplément à l’instruction sur les moyens de pourvoir à la disette des fourrages, publiée par ordre du Roi le 31 mai 1785 (Instruction on the means of compensating for the food shortage with fodder, and of increasing the subsistence of cattle, Supplement to the instruction on the means of providing for the food shortage with fodder, published by order of King on 31 May 1785).
- (with Guyton de Morveau, Claude-Louis Berthollet, Antoine Fourcroy) Méthode de nomenclature chimique (Paris: Chez Cuchet, 1787)
- (with Fourcroy, Morveau, Cadet, Baumé, d’Arcet, and Sage) Nomenclature chimique, ou synonymie ancienne et moderne, pour servir à l’intelligence des auteurs. (Paris: Chez Cuchet, 1789)
- Traité élémentaire de chimie, présenté dans un ordre nouveau et d’après les découvertes modernes (Paris: Chez Cuchet, 1789; Bruxelles: Cultures et Civilisations, 1965) (lit. Elementary Treatise on Chemistry, presented in a new order and alongside modern discoveries)
- (with Pierre-Simon Laplace) “Mémoire sur la chaleur,” Mémoires de l’Académie des sciences (1780), pp. 355–408.
- Mémoire contenant les expériences faites sur la chaleur, pendant l’hiver de 1783 à 1784, par P.S. de Laplace & A. K. Lavoisier (1792)
- Mémoires de Physique et de Chimie, de la Société d’Arcueil (1805: posthumous)
In translation
- Essays Physical and Chemical (London: for Joseph Johnson, 1776; London: Frank Cass and Company Ltd., 1970) translation by Thomas Henry of Opuscules physiques et chimiques
- The Art of Manufacturing Alkaline Salts and Potashes, Published by Order of His Most Christian Majesty, and approved by the Royal Academy of Sciences (1784) trans. by Charles Williamos of L’art de fabriquer le salin et la potasse
- (with Pierre-Simon Laplace) Memoir on Heat: Read to the Royal Academy of Sciences, 28 June 1783, by Messrs. Lavoisier & De La Place of the same Academy. (New York: Neale Watson Academic Publications, 1982) trans. by Henry Guerlac of Mémoire sur la chaleur
- Essays, on the Effects Produced by Various Processes On Atmospheric Air; With A Particular View To An Investigation Of The Constitution Of Acids, trans. Thomas Henry (London: Warrington, 1783) collects these essays:
- “Experiments on the Respiration of Animals, and on the Changes effected on the Air in passing through their Lungs.” (Read to the Académie des Sciences, 3 May 1777)
- “On the Combustion of Candles in Atmospheric Air and in Dephlogistated Air.” (Communicated to the Académie des Sciences, 1777)
- “On the Combustion of Kunckel’s Phosphorus.”
- “On the Existence of Air in the Nitrous Acid, and on the Means of decomposing and recomposing that Acid.”
- “On the Solution of Mercury in Vitriolic Acid.”
- “Experiments on the Combustion of Alum with Phlogistic Substances, and on the Changes effected on Air in which the Pyrophorus was burned.”
- “On the Vitriolisation of Martial Pyrites.”
- “General Considerations on the Nature of Acids, and on the Principles of which they are composed.”
- “On the Combination of the Matter of Fire with Evaporable Fluids; and on the Formation of Elastic Aëriform Fluids.”
- “Reflections on Phlogiston”, translation by Nicholas W. Best of “Réflexions sur le phlogistique, pour servir de suite à la théorie de la combustion et de la calcination” (read to the Académie Royale des Sciences over two nights, 28 June and 13 July 1783). Published in two parts:
- Best, Nicholas W. (2015). “Lavoisier’s “Reflections on phlogiston” I: Against phlogiston theory”. Foundations of Chemistry. 17 (2): 361–378. doi:10.1007/s10698-015-9220-5. S2CID 170422925.
- Best, Nicholas W. (2016). “Lavoisier’s “Reflections on phlogiston” II: On the nature of heat”. Foundations of Chemistry. 18 (1): 3–13. doi:10.1007/s10698-015-9236-x. S2CID 94677080.
- Method of chymical nomenclature: proposed by Messrs. De Moreau, Lavoisier, Bertholet, and De Fourcroy (1788) Dictionary
- Elements of Chemistry, in a New Systematic Order, Containing All the Modern Discoveries (Edinburgh: William Creech, 1790; New York: Dover, 1965) translation by Robert Kerr of Traité élémentaire de chimie. ISBN 978-0-486-64624-4 (Dover).
- 1799 edition
- 1802 edition: volume 1, volume 2
- Some illustrations from 1793 edition
- Some more illustrations from the Science History Institute
- More illustrations (from Collected Works) from the Science History Institute
Notes
- “Lavoisier, Antoine Laurent”. Lexico UK English Dictionary. Oxford University Press. Archived from the original on 23 April 2021.
- “Lavoisier”. Collins English Dictionary. HarperCollins. Retrieved 30 July 2019.
- “Lavoisier”. Merriam-Webster.com Dictionary. Retrieved 30 July 2019.
- (in French) Lavoisier, le parcours d’un scientifique révolutionnaire CNRS (Centre National de la Recherche Scientifique)
- Schwinger, Julian (1986). Einstein’s Legacy. New York: Scientific American Library. p. 93. ISBN 978-0-7167-5011-6.
- In his table of the elements, Lavoisier listed five “salifiable earths” (i.e., ores that could be made to react with acids to produce salts (salis = salt, in Latin)): chaux (calcium oxide), magnésie (magnesia, magnesium oxide), baryte (barium sulfate), alumine (alumina, aluminium oxide), and silice (silica, silicon dioxide). About these “elements”, Lavoisier speculates: “We are probably only acquainted as yet with a part of the metallic substances existing in nature, as all those which have a stronger affinity to oxygen than carbon possesses, are incapable, hitherto, of being reduced to a metallic state, and consequently, being only presented to our observation under the form of oxyds, are confounded with earths. It is extremely probable that barytes, which we have just now arranged with earths, is in this situation; for in many experiments it exhibits properties nearly approaching to those of metallic bodies. It is even possible that all the substances we call earths may be only metallic oxyds, irreducible by any hitherto known process.” – from p. 218 of: Lavoisier with Robert Kerr, trans., Elements of Chemistry, …, 4th ed. (Edinburgh, Scotland: William Creech, 1799). (The original passage appears in: Lavoisier, Traité Élémentaire de Chimie, … (Paris, France: Cuchet, 1789), vol. 1, p. 174.)
- Schama, Simon (1989). Citizens: A Chronicle of the French Revolution. Alfred A Knopf. p. 73.
- Herbermann, Charles, ed. (1913). “Antoine-Laurent Lavoisier” . Catholic Encyclopedia. New York: Robert Appleton Company.
- Chisholm, Hugh, ed. (1911). “Lavoisier, Antoine Laurent” . Encyclopædia Britannica. Vol. 16 (11th ed.). Cambridge University Press. p. 295.
- Yount, Lisa (2008). Antoine Lavoisier : founder of modern chemistry. Berkeley Heights, NJ: Enslow Publishers. p. 115. ISBN 978-0-7660-3011-4. Retrieved 25 July 2016.
- Duveen, Dennis I. (1965). Supplement to a bibliography of the works of Antoine Laurent Lavoisier, 1743–1794. London: Dawsons.
- McKie, Douglas (1935). Bibliographic Details Antoine Lavoisier, the father of modern chemistry, by Douglas McKie … With an introduction by F.G. Donnan. London: V. Gollancz ltd.
- Bibliographic Details Lavoisier in perspective / edited by Marco Beretta. Munich: Deutsches Museum. 2005.
- Bell, Madison Smart (2005). Lavoisier in the year one. New York: W.W. Norton.
- McKie, Douglas (1952). Antoine Lavoisier: scientist, economist, social reformer. New York: Schuman.
- Citizens, Simon Schama. Penguin 1989 p. 236
- Eagle, Cassandra T.; Jennifer Sloan (1998). “Marie Anne Paulze Lavoisier: The Mother of Modern Chemistry”. The Chemical Educator. 3 (5): 1–18. CiteSeerX 10.1.1.472.7513. doi:10.1007/s00897980249a. S2CID 97557390.
- Donovan, Arthur (1996). Antoine Lavoisier: Science, Administration, and Revolution. Cambridge: Cambridge University Press. p. 273. ISBN 978-0-521-56672-8.
- Jean-Pierre Poirier (1998). Lavoisier: Chemist, Biologist, Economist. University of Pennsylvania Press. pp. 24–26. ISBN 978-0-8122-1649-3.
- W.R. Aykroyd (12 May 2014). Three Philosophers: Lavoisier, Priestley and Cavendish. Elsevier Science. pp. 168–170. ISBN 978-1-4831-9445-5.
- Arthur Donovan (11 April 1996). Antoine Lavoisier: Science, Administration and Revolution. Cambridge University Press. pp. 123–125. ISBN 978-0-521-56672-8.
- Citizens, Simon Schama, Penguin 1989 p. 313
- Dutton, William S. (1942), Du Pont: One Hundred and Forty Years, Charles Scribner’s Sons, LCCN 42011897.
- Chronicle of the French Revolution, Jacques Legrand, Longman 1989, p. 216 ISBN 0-582-05194-0
- Companion to the French Revolution, John Paxton, Facts on File Publications 1988, p. 120
- A Cultural History of the French Revolution, Emmet Kennedy, Yale University Press 1989, p. 193
- Chronicle of the French Revolution, Jacques Legrand, Longman 1989, p. 204 ISBN 0-582-05194-0
- Chronicle of the French Revolution, Jacques Legrand, Longman 1989, p. 356 ISBN 0-582-05194-0
- Chronicle of the French Revolution, Jacques Legrand, Longman 1989 ISBN 0-582-05194-0[page needed]
- O’Connor, J.J.; Robertson, E.F. (26 September 2006). “Joseph-Louis Lagrange”. Archived from the original on 2 May 2006. Retrieved 20 April 2006.
In September 1793 a law was passed ordering the arrest of all foreigners born in enemy countries and all their property to be confiscated. Lavoisier intervened on behalf of Lagrange, who certainly fell under the terms of the law. On 8 May 1794, after a trial that lasted less than a day, a revolutionary tribunal condemned Lavoisier and 27 others to death. Lagrange said on the death of Lavoisier, who was guillotined on the afternoon of the day of his trial
- Chronicle of the French Revolution, Longman 1989 p. 202 ISBN 0-582-05194-0
- “Today in History: 1794: Antoine Lavoisier, the father of modern chemistry, is executed on the guillotine during France’s Reign of Terror”. Archived from the original on 15 June 2013.
- Commenting on this quotation, Denis Duveen, an English expert on Lavoiser and a collector of his works, wrote that “it is pretty certain that it was never uttered”. For Duveen’s evidence, see the following: Duveen, Denis I. (February 1954). “Antoine Laurent Lavoisier and the French Revolution”. Journal of Chemical Education. 31 (2): 60–65. Bibcode:1954JChEd..31…60D. doi:10.1021/ed031p60
- Delambre, Jean-Baptiste (1867). Œuvres de Lagrange (in French). Gauthier-Villars. 15–57 – via Wikisource.
- Guerlac, Henry (1973). Antoine-Laurent Lavoisier – Chemist and Revolutionary. New York: Charles Scribner’s Sons. p. 130.
- (In French) M.-A. Paulze, épouse et collaboratrice de Lavoisier, Vesalius, VI, 2, 105–113, 2000, p. 110. (PDF)
- Guerlac, Henry (2019). “Lavoisier—the Crucial Year: The Background and Origin of His First Experiments on Combustion in 1772”. Cornell University Press. doi:10.7298/GX84-1A09.
- Lavoisier, Antoine (1777) “Mémoire sur la combustion en général” Archived 17 June 2013 at the Wayback Machine (“On Combustion in General”). Mémoires de l’Académie des sciences. English translation
- Petrucci R.H., Harwood W.S. and Herring F.G., General Chemistry (8th ed. Prentice-Hall 2002), p. 34
- “An Historical Note on the Conservation of Mass”.
- Duveen, Denis; Klickstein, Herbert (September 1954). “The Introduction of Lavoisier’s Chemical Nomenclature into America”. The History of Science Society. 45 (3).
- “Traité élémentaire de chimie : Présenté dans un ordre nouveau et d’après les découvertes modernes ; avec figures”. A Paris, Chez Cuchet, libraire, rue et Hôtel Serpente. 1789.
- Holleman, A. F.; Wiberg, Egon; Wiberg, Nils (2001). Inorganic Chemistry. Academic Press. p. 17. ISBN 978-0-12-352651-9.
- Golinski, Jan (1994). “Precision instruments and the demonstrative order of proof in Lavoisier’s chemistry” (PDF). Osiris. 9: 30–47. doi:10.1086/368728. JSTOR 301997. S2CID 95978870.
- Kirwan, Essay on Phlogiston, viii, xi.
- Lavoisier, Antoine (1778) “Considérations générales sur la nature des acides” Archived 17 June 2013 at the Wayback Machine (“General Considerations on the Nature of Acids”). Mémoires de l’Académie des sciences. lavoisier.cnrs.fr
- Gillispie, Charles Coulston (1960). The Edge of Objectivity: An Essay in the History of Scientific Ideas. Princeton University Press. p. 228. ISBN 0-691-02350-6.
- Lavoisier and Meusnier, “Développement” (cit. n. 27), pp. 205–209; cf. Holmes, Lavoisier (cn. 8), p. 237.
- See the “Advertisement,” p. vi of Kerr’s translation, and pp. xxvi–xxvii, xxviii of Douglas McKie’s introduction to the Dover edition.
- Is a Calorie a Calorie? American Journal of Clinical Nutrition, Vol. 79, No. 5, 899S–906S, May 2004
- Levere, Trevor (2001). Transforming Matter. Maryland: The Johns Hopkins University Press. pp. 72–73. ISBN 978-0-8018-6610-4.
- Gillespie, Charles C. (1996), Foreword to Lavoisier by Jean-Pierre Poirier, University of Pennsylvania Press, English Edition.
- Beretta, Marco (8 June 2022). The Arsenal of Eighteenth-Century Chemistry:The Laboratories of Antoine Laurent Lavoisier (1743-1794). Koninklijke Brill. p. 107. ISBN 9789004511217. Archived from the original on 24 December 2023. Retrieved 24 December 2023.
- “Place name detail: Mount Lavoisier”. New Zealand Gazetteer. New Zealand Geographic Board. Retrieved 21 August 2022.
- “APS Member History”. search.amphilsoc.org. Retrieved 28 May 2021.
- “Antoine-Laurent Lavoisier: The Chemical Revolution”. National Historic Chemical Landmarks. American Chemical Society. Archived from the original on 23 February 2013. Retrieved 25 March 2013.
- Guyton de Morveau, Louis Bernard; Lavoisier, Antoine Laurent; Berthollet, Claude-Louis; Fourcroy, Antoine-François de (1787). Méthode de Nomenclature Chimique. Paris, France: Chez Cuchet (Sous le Privilége de l’Académie des Sciences).
- “2015 Awardees”. American Chemical Society, Division of the History of Chemistry. University of Illinois at Urbana-Champaign School of Chemical Sciences. 2015. Retrieved 1 July 2016.
- “Citation for Chemical Breakthrough Award” (PDF). American Chemical Society, Division of the History of Chemistry. University of Illinois at Urbana-Champaign School of Chemical Sciences. 2015. Archived (PDF) from the original on 9 October 2022. Retrieved 1 July 2016.
- “Société Chimique de France”. www.societechimiquedefrance.fr. Archived from the original on 29 March 2019. Retrieved 28 March 2019.
- “International Society for Biological Calorimetry (ISBC) – About ISBC_”. biocalorimetry.ucoz.org. Retrieved 28 March 2019.
- workflow-process-service. “The Lavoisier Medal honors exceptional scientists and engineers | DuPont USA”. www.dupont.com. Retrieved 28 March 2019.
- “Le Prix Franklin–Lavoiser2018 a été décerné au Comité Lavoisier”. La Gazette du Laboratoire. 20 June 2018. Retrieved 15 January 2019.
- “Franklin-Lavoisier Prize”. Science History Institute. Archived from the original on 26 March 2020. Retrieved 26 March 2020.
- See Denis I. Duveen and Herbert S. Klickstein, “The “American” Edition of Lavoisier’s L’art de fabriquer le salin et la potasse,” The William and Mary Quarterly, Third Series 13:4 (October 1956), 493–498.
Further reading
- Herbermann, Charles, ed. (1913). “Antoine-Laurent Lavoisier” . Catholic Encyclopedia. New York: Robert Appleton Company.
- Bailly, J.-S., “Secret Report on Mesmerism or Animal Magnetism”, International Journal of Clinical and Experimental Hypnosis, Vol. 50, No. 4, (October 2002), pp. 364–368. doi:10.1080/00207140208410110
- Berthelot, M. (1890). La révolution chimique: Lavoisier. Paris: Alcan.
- Catalogue of Printed Works by and Memorabilia of Antoine Laurent Lavoisier, 1743–1794… Exhibited at the Grolier Club (New York, 1952).
- Daumas, M. (1955). Lavoisier, théoricien et expérimentateur. Paris: Presses Universitaires de France.
- Donovan, Arthur (1993). Antoine Lavoisier: Science, Administration, and Revolution. Cambridge, England: Cambridge University Press.
- Duveen, D.I. and H.S. Klickstein, A Bibliography of the Works of Antoine Laurent Lavoisier, 1743–1794 (London, 1954)
- Franklin, B., Majault, M.J., Le Roy, J.B., Sallin, C.L., Bailly, J.-S., d’Arcet, J., de Bory, G., Guillotin, J.-I. & Lavoisier, A., “Report of The Commissioners charged by the King with the Examination of Animal Magnetism”, International Journal of Clinical and Experimental Hypnosis, Vol.50, No.4, (October 2002), pp. 332–363. doi:10.1080/00207140208410109
- Grey, Vivian (1982). The Chemist Who Lost His Head: The Story of Antoine Lavoisier. Coward, McCann & Geoghegan, Inc. ISBN 9780698205598.
- Guerlac, Henry (1961). Lavoisier – The Crucial Year. Ithaca, New York: Cornell University Press.
- Holmes, Frederic Lawrence (1985). Lavoisier and the Chemistry of Life. Madison, Wisconsin: University of Wisconsin Press.
- Holmes, Frederic Lawrence (1998). Antoine Lavoisier – The Next Crucial Year, or the Sources of his Quantitative Method in Chemistry. Princeton University Press.
- Jackson, Joe (2005). A World on Fire: A Heretic, An Aristocrat And The Race to Discover Oxygen. Viking.
- Johnson, Horton A. (2008). “Revolutionary Instruments, Lavoisier’s Tools as Objets d’Art”. Chemical Heritage Magazine. 26 (1): 30–35.
- Kelly, Jack (2004). Gunpowder: Alchemy, Bombards, & Pyrotechnics. Basic Books. ISBN 978-0-465-03718-6.
- McKie, Douglas (1935). Antoine Lavoisier: The Father of Modern Chemistry. Philadelphia: J. B. Lippincott & Co.
- McKie, Douglas (1952). Antoine Lavoisier: Scientist, Economist, Social Reformer. New York: Henry Schuman.
- Poirier, Jean-Pierre (1996). Lavoisier (English ed.). University of Pennsylvania Press.
- Scerri, Eric (2007). The Periodic Table: Its Story and Its Significance. Oxford University Press. ISBN 978-0-19-530573-9.
- Smartt Bell, Madison (2005). Lavoisier in the Year One: The Birth of a New Science in an Age of Revolution. Atlas Books, W.W. Norton.
Tab Content
Tab Content
Priestley is credited with his independent discovery of oxygen by the thermal decomposition of mercuric oxide, having isolated it in 1774. During his lifetime, Priestley’s considerable scientific reputation rested on his invention of carbonated water, his writings on electricity, and his discovery of several “airs” (gases), the most famous being what Priestley dubbed “dephlogisticated air” (oxygen). Priestley’s determination to defend phlogiston theory and to reject what would become the chemical revolution eventually left him isolated within the scientific community.
Biography
Joseph Priestley FRS (24 March 1733 – 6 February 1804) was an English chemist, natural philosopher, separatist theologian, grammarian, multi-subject educator, and liberal political theorist. He published over 150 works, and conducted experiments in several areas of science.
Priestley’s science was integral to his theology, and he consistently tried to fuse Enlightenment rationalism with Christian theism. In his metaphysical texts, Priestley attempted to combine theism, materialism, and determinism, a project that has been called “audacious and original”. He believed that a proper understanding of the natural world would promote human progress and eventually bring about the Christian millennium. Priestley, who strongly believed in the free and open exchange of ideas, advocated toleration and equal rights for religious Dissenters, which also led him to help found Unitarianism in England. The controversial nature of Priestley’s publications, combined with his outspoken support of the American Revolution and later the French Revolution, aroused public and governmental contempt; eventually forcing him to flee in 1791, first to London and then to the United States, after a mob burned down his Birmingham home and church. He spent his last ten years in Northumberland County, Pennsylvania.
A scholar and teacher throughout his life, Priestley made significant contributions to pedagogy, including the publication of a seminal work on English grammar and books on history; he prepared some of the most influential early timelines. The educational writings were among Priestley’s most popular works. Arguably his metaphysical works, however, had the most lasting influence, as now considered primary sources for utilitarianism by philosophers such as Jeremy Bentham, John Stuart Mill, and Herbert Spencer.
Early life and education (1733–1755)
Priestley was born in Birstall (near Batley) in the West Riding of Yorkshire, to an established English Dissenting family who did not conform to the Church of England. He was the oldest of six children born to Mary Swift and Jonas Priestley, a finisher of cloth. Priestley was sent to live with his grandfather around the age of one. He returned home five years later, after his mother died. When his father remarried in 1741, Priestley went to live with his aunt and uncle, the wealthy and childless Sarah (d. 1764) and John Keighley, 3 miles (4.8 km) from Fieldhead.
Priestley was a precocious child – at the age of four, he could flawlessly recite all 107 questions and answers of the Westminster Shorter Catechism – and his aunt sought the best education for him, intending him to enter ministry. During his youth, Priestley attended local schools, where he learned Greek, Latin, and Hebrew.
Around 1749, Priestley became seriously ill and believed he was dying. Raised as a devout Calvinist, he believed a conversion experience was necessary for salvation, but doubted he had had one. This emotional distress eventually led him to question his theological upbringing, causing him to reject election and to accept universal salvation. As a result, the elders of his home church, the Independent Upper Chapel of Heckmondwike, near Leeds, refused him admission as a full member.
Priestley’s illness left him with a permanent stutter and he gave up any thoughts of entering the ministry at that time. In preparation for joining a relative in trade in Lisbon, he studied French, Italian, and German in addition to Aramaic, and Arabic. He was tutored by the Reverend George Haggerstone, who first introduced him to higher mathematics, natural philosophy, logic, and metaphysics through the works of Isaac Watts, Willem ‘s Gravesande, and John Locke.
Daventry Academy
Priestley eventually decided to return to his theological studies and, in 1752, matriculated at Daventry, a Dissenting academy. Because he was already widely read, Priestley was allowed to omit the first two years of coursework. He continued his intense study; this, together with the liberal atmosphere of the school, shifted his theology further leftward and he became a Rational Dissenter. Abhorring dogma and religious mysticism, Rational Dissenters emphasised rational analysis of the natural world and the Bible.
Priestley later wrote that the book that influenced him the most, save the Bible, was David Hartley’s Observations on Man (1749). Hartley’s psychological, philosophical, and theological treatise postulated a material theory of mind. Hartley aimed to construct a Christian philosophy in which both religious and moral “facts” could be scientifically proven, a goal that would occupy Priestley for his entire life. In his third year at Daventry, Priestley committed himself to the ministry, which he described as “the noblest of all professions”.
Needham Market and Nantwich (1755–1761)
Robert Schofield, Priestley’s major modern biographer, describes his first “call” in 1755 to the Dissenting parish in Needham Market, Suffolk, as a “mistake” for both Priestley and the congregation. Priestley yearned for urban life and theological debate, whereas Needham Market was a small, rural town with a congregation wedded to tradition. Attendance and donations dropped sharply when they discovered the extent of his heterodoxy. Although Priestley’s aunt had promised her support if he became a minister, she refused any further assistance when she realised he was no longer a Calvinist. To earn extra money, Priestley proposed opening a school, but local families informed him that they would refuse to send their children. He also presented a series of scientific lectures titled “Use of the Globes” that was more successful.
Priestley’s Daventry friends helped him obtain another position and in 1758 he moved to Nantwich, Cheshire, living at Sweetbriar Hall in the town’s Hospital Street; his time there was happier. The congregation cared less about Priestley’s heterodoxy and he successfully established a school. Unlike many schoolmasters of the time, Priestley taught his students natural philosophy and even bought scientific instruments for them. Appalled at the quality of the available English grammar books, Priestley wrote his own: The Rudiments of English Grammar (1761). His innovations in the description of English grammar, particularly his efforts to dissociate it from Latin grammar, led 20th-century scholars to describe him as “one of the great grammarians of his time”. After the publication of Rudiments and the success of Priestley’s school, Warrington Academy offered him a teaching position in 1761.
Warrington Academy (1761–1767)
In 1761, Priestley moved to Warrington in Cheshire and assumed the post of tutor of modern languages and rhetoric at the town’s Dissenting academy, although he would have preferred to teach mathematics and natural philosophy. He fitted in well at Warrington, and made friends quickly. These included the doctor and writer John Aikin, his sister the children’s author Anna Laetitia Aikin, and the potter and businessman Josiah Wedgwood. Wedgwood met Priestley in 1762, after a fall from his horse. Wedgwood and Priestley met rarely, but exchanged letters, advice on chemistry, and laboratory equipment. Wedgwood eventually created a medallion of Priestley in cream-on-blue jasperware.
On 23 June 1762, Priestley married Mary Wilkinson of Wrexham. Of his marriage, Priestley wrote:
This proved a very suitable and happy connexion, my wife being a woman of an excellent understanding, much improved by reading, of great fortitude and strength of mind, and of a temper in the highest degree affectionate and generous; feeling strongly for others, and little for herself. Also, greatly excelling in every thing relating to household affairs, she entirely relieved me of all concern of that kind, which allowed me to give all my time to the prosecution of my studies, and the other duties of my station.
On 17 April 1763, they had a daughter, whom they named Sarah after Priestley’s aunt.
Educator and historian
All of the books Priestley published while at Warrington emphasised the study of history; Priestley considered it essential for worldly success as well as religious growth. He wrote histories of science and Christianity in an effort to reveal the progress of humanity and, paradoxically, the loss of a pure, “primitive Christianity”.
In his Essay on a Course of Liberal Education for Civil and Active Life (1765), Lectures on History and General Policy (1788), and other works, Priestley argued that the education of the young should anticipate their future practical needs. This principle of utility guided his unconventional curricular choices for Warrington’s aspiring middle-class students. He recommended modern languages instead of classical languages and modern rather than ancient history. Priestley’s lectures on history were particularly revolutionary; he narrated a providentialist and naturalist account of history, arguing that the study of history furthered the comprehension of God’s natural laws. Furthermore, his millennial perspective was closely tied to his optimism regarding scientific progress and the improvement of humanity. He believed that each age would improve upon the previous and that the study of history allowed people to perceive and to advance this progress. Since the study of history was a moral imperative for Priestley, he also promoted the education of middle-class women, which was unusual at the time. Some scholars of education have described Priestley as the most important English writer on education between the 17th-century John Locke and the 19th-century Herbert Spencer. Lectures on History was well received and was employed by many educational institutions, such as New College at Hackney, Brown, Princeton, Yale, and Cambridge. Priestley designed two Charts to serve as visual study aids for his Lectures. These charts are in fact timelines; they have been described as the most influential timelines published in the 18th century. Both were popular for decades, and the trustees of Warrington were so impressed with Priestley’s lectures and charts that they arranged for the University of Edinburgh to grant him a Doctor of Law degree in 1764. During this period Priestley also regularly delivered lectures on rhetoric that were later published in 1777 as A Course of Lectures on Oratory and Criticism.
History of electricity
The intellectually stimulating atmosphere of Warrington, often called the “Athens of the North” (of England) during the 18th century, encouraged Priestley’s growing interest in natural philosophy. He gave lectures on anatomy and performed experiments regarding temperature with another tutor at Warrington, his friend John Seddon. Despite Priestley’s busy teaching schedule, he decided to write a history of electricity. Friends introduced him to the major experimenters in the field in Britain—John Canton, William Watson, Timothy Lane, and the visiting Benjamin Franklin who encouraged Priestley to perform the experiments he wanted to include in his history. Priestley also consulted with Franklin during the latter’s kite experiments. In the process of replicating others’ experiments, Priestley became intrigued by unanswered questions and was prompted to undertake experiments of his own design. (Impressed with his Charts and the manuscript of his history of electricity, Canton, Franklin, Watson, and Richard Price nominated Priestley for a fellowship in the Royal Society; he was accepted in 1766.)
In 1767, the 700-page The History and Present State of Electricity was published to positive reviews. The first half of the text is a history of the study of electricity to 1766; the second and more influential half is a description of contemporary theories about electricity and suggestions for future research. The volume also contains extensive comments on Priestley’s views that scientific inquiries be presented with all reasoning in one’s discovery path, including false leads and mistakes. He contrasted his narrative approach with Newton’s analytical proof-like approach which did not facilitate future researchers to continue the inquiry. Priestley reported some of his own discoveries in the second section, such as the conductivity of charcoal and other substances and the continuum between conductors and non-conductors. This discovery overturned what he described as “one of the earliest and universally received maxims of electricity”, that only water and metals could conduct electricity. This and other experiments on the electrical properties of materials and on the electrical effects of chemical transformations demonstrated Priestley’s early and ongoing interest in the relationship between chemical substances and electricity. Based on experiments with charged spheres, Priestley was among the first to propose that electrical force followed an inverse-square law, similar to Newton’s law of universal gravitation. He did not generalise or elaborate on this, and the general law was enunciated by French physicist Charles-Augustin de Coulomb in the 1780s.
Priestley’s strength as a natural philosopher was qualitative rather than quantitative and his observation of “a current of real air” between two electrified points would later interest Michael Faraday and James Clerk Maxwell as they investigated electromagnetism. Priestley’s text became the standard history of electricity for over a century; Alessandro Volta (who later invented the battery), William Herschel (who discovered infrared radiation), and Henry Cavendish (who discovered hydrogen) all relied upon it. Priestley wrote a popular version of the History of Electricity for the general public titled A Familiar Introduction to the Study of Electricity (1768). He marketed the book with his brother Timothy, but unsuccessfully.
Leeds (1767–1773)
Perhaps prompted by Mary Priestley’s ill health, or financial problems, or a desire to prove himself to the community that had rejected him in his childhood, Priestley moved with his family from Warrington to Leeds in 1767, and he became Mill Hill Chapel’s minister. Two sons were born to the Priestleys in Leeds: Joseph junior on 24 July 1768 and William three years later. Theophilus Lindsey, a rector at Catterick, Yorkshire, became one of Priestley’s few friends in Leeds, of whom he wrote: “I never chose to publish any thing of moment relating to theology, without consulting him.” Although Priestley had extended family living around Leeds, it does not appear that they communicated. Schofield conjectures that they considered him a heretic. Each year Priestley travelled to London to consult with his close friend and publisher, Joseph Johnson, and to attend meetings of the Royal Society.
Minister of Mill Hill Chapel
When Priestley became its minister, Mill Hill Chapel was one of the oldest and most respected Dissenting congregations in England; however, during the early 18th century the congregation had fractured along doctrinal lines, and was losing members to the charismatic Methodist movement. Priestley believed that by educating the young, he could strengthen the bonds of the congregation.
In his three-volume Institutes of Natural and Revealed Religion (1772–74), Priestley outlined his theories of religious instruction. More importantly, he laid out his belief in Socinianism. The doctrines he explicated would become the standards for Unitarians in Britain. This work marked a change in Priestley’s theological thinking that is critical to understanding his later writings—it paved the way for his materialism and necessitarianism (the belief that a divine being acts in accordance with necessary metaphysical laws).
Priestley’s major argument in the Institutes was that the only revealed religious truths that could be accepted were those that matched one’s experience of the natural world. Because his views of religion were deeply tied to his understanding of nature, the text’s theism rested on the argument from design. The Institutes shocked and appalled many readers, primarily because it challenged basic Christian orthodoxies, such as the divinity of Christ and the miracle of the Virgin Birth. Methodists in Leeds penned a hymn asking God to “the Unitarian fiend expel / And chase his doctrine back to Hell.” Priestley wanted to return Christianity to its “primitive” or “pure” form by eliminating the “corruptions” which had accumulated over the centuries. The fourth part of the Institutes, An History of the Corruptions of Christianity, became so long that he was forced to issue it separately in 1782. Priestley believed that the Corruptions was “the most valuable” work he ever published. In demanding that his readers apply the logic of the emerging sciences and comparative history to the Bible and Christianity, he alienated religious and scientific readers alike—scientific readers did not appreciate seeing science used in the defence of religion and religious readers dismissed the application of science to religion.
Religious controversialist
Priestley engaged in numerous political and religious pamphlet wars. According to Schofield, “he entered each controversy with a cheerful conviction that he was right, while most of his opponents were convinced, from the outset, that he was willfully and maliciously wrong. He was able, then, to contrast his sweet reasonableness to their personal rancor”, but as Schofield points out Priestley rarely altered his opinion as a result of these debates. While at Leeds he wrote controversial pamphlets on the Lord’s Supper and on Calvinist doctrine; thousands of copies were published, making them some of Priestley’s most widely read works.
Priestley founded the Theological Repository in 1768, a journal committed to the open and rational inquiry of theological questions. Although he promised to print any contribution, only like-minded authors submitted articles. He was therefore obliged to provide much of the journal’s content himself (this material became the basis for many of his later theological and metaphysical works). After only a few years, due to a lack of funds, he was forced to cease publishing the journal. He revived it in 1784 with similar results.
Defender of Dissenters and political philosopher
Many of Priestley’s political writings supported the repeal of the Test and Corporation Acts, which restricted the rights of Dissenters. They could not hold political office, serve in the armed forces, or attend Oxford and Cambridge unless they subscribed to the Thirty-nine Articles of the Church of England. Dissenters repeatedly petitioned Parliament to repeal the Acts, arguing that they were being treated as second-class citizens.
Priestley’s friends, particularly other Rational Dissenters, urged him to publish a work on the injustices experienced by Dissenters; the result was his Essay on the First Principles of Government (1768). An early work of modern liberal political theory and Priestley’s most thorough treatment of the subject, it—unusually for the time—distinguished political rights from civil rights with precision and argued for expansive civil rights. Priestley identified separate private and public spheres, contending that the government should have control only over the public sphere. Education and religion, in particular, he maintained, were matters of private conscience and should not be administered by the state. Priestley’s later radicalism emerged from his belief that the British government was infringing upon these individual freedoms.
Priestley also defended the rights of Dissenters against the attacks of William Blackstone, an eminent legal theorist, whose Commentaries on the Laws of England (1765–69) had become the standard legal guide. Blackstone’s book stated that dissent from the Church of England was a crime and that Dissenters could not be loyal subjects. Furious, Priestley lashed out with his Remarks on Dr. Blackstone’s Commentaries (1769), correcting Blackstone’s interpretation of the law, his grammar (a highly politicised subject at the time), and history. Blackstone, chastened, altered subsequent editions of his Commentaries: he rephrased the offending passages and removed the sections claiming that Dissenters could not be loyal subjects, but he retained his description of Dissent as a crime.
Natural philosopher: electricity, Optics, and carbonated water
Although Priestley claimed that natural philosophy was only a hobby, he took it seriously. In his History of Electricity, he described the scientist as promoting the “security and happiness of mankind”. Priestley’s science was eminently practical and he rarely concerned himself with theoretical questions; his model was his close friend, Benjamin Franklin. When he moved to Leeds, Priestley continued his electrical and chemical experiments (the latter aided by a steady supply of carbon dioxide from a neighbouring brewery). Between 1767 and 1770, he presented five papers to the Royal Society from these initial experiments; the first four papers explored coronal discharges and other phenomena related to electrical discharge, while the fifth reported on the conductivity of charcoals from different sources. His subsequent experimental work focused on chemistry and pneumatics.
Priestley published the first volume of his projected history of experimental philosophy, The History and Present State of Discoveries Relating to Vision, Light and Colours (referred to as his Optics), in 1772. He paid careful attention to the history of optics and presented excellent explanations of early optics experiments, but his mathematical deficiencies caused him to dismiss several important contemporary theories. He followed the (corpuscular) particle theory of light, influenced by the works of Reverend John Rowning and others. Furthermore, he did not include any of the practical sections that had made his History of Electricity so useful to practising natural philosophers. Unlike his History of Electricity, it was not popular and had only one edition, although it was the only English book on the topic for 150 years. The hastily written text sold poorly; the cost of researching, writing, and publishing the Optics convinced Priestley to abandon his history of experimental philosophy.
| External audio | |
|---|---|
Priestley was considered for the position of astronomer on James Cook’s second voyage to the South Seas, but was not chosen. Still, he contributed in a small way to the voyage: he provided the crew with a method for making carbonated water, which he erroneously speculated might be a cure for scurvy. He then published a pamphlet with Directions for Impregnating Water with Fixed Air (1772). Priestley did not exploit the commercial potential of carbonated water, but others such as J. J. Schweppe made fortunes from it. For his discovery of carbonated water Priestley has been labelled “the father of the soft drink”, with the beverage company Schweppes regarding him as “the father of our industry”. In 1773, the Royal Society recognised Priestley’s achievements in natural philosophy by awarding him the Copley Medal.
Priestley’s friends wanted to find him a more financially secure position. In 1772, prompted by Richard Price and Benjamin Franklin, Lord Shelburne wrote to Priestley asking him to direct the education of his children and to act as his general assistant. Although Priestley was reluctant to sacrifice his ministry, he accepted the position, resigning from Mill Hill Chapel on 20 December 1772, and preaching his last sermon on 16 May 1773.
Calne (1773–1780)
In 1773, the Priestleys moved to Calne in Wiltshire, and a year later Lord Shelburne and Priestley took a tour of Europe. According to Priestley’s close friend Theophilus Lindsey, Priestley was “much improved by this view of mankind at large”. Upon their return, Priestley easily fulfilled his duties as librarian and tutor. The workload was intentionally light, allowing him time to pursue his scientific investigations and theological interests. Priestley also became a political adviser to Shelburne, gathering information on parliamentary issues and serving as a liaison between Shelburne and the Dissenting and American interests. When the Priestleys’ third son was born on 24 May 1777, they named him Henry at the lord’s request.
Materialist philosopher
Priestley wrote his most important philosophical works during his years with Lord Shelburne. In a series of major metaphysical texts published between 1774 and 1780—An Examination of Dr. Reid’s Inquiry into the Human Mind (1774), Hartley’s Theory of the Human Mind on the Principle of the Association of Ideas (1775), Disquisitions relating to Matter and Spirit (1777), The Doctrine of Philosophical Necessity Illustrated (1777), and Letters to a Philosophical Unbeliever (1780)—he argues for a philosophy that incorporates four concepts: determinism, materialism, causation, and necessitarianism. By studying the natural world, he argued, people would learn how to become more compassionate, happy, and prosperous.
Priestley strongly suggested that there is no mind-body duality, and put forth a materialist philosophy in these works; that is, one founded on the principle that everything in the universe is made of matter that we can perceive. He also contended that discussing the soul is impossible because it is made of a divine substance, and humanity cannot perceive the divine. Despite his separation of the divine from the mortal, this position shocked and angered many of his readers, who believed that such a duality was necessary for the soul to exist.
Responding to Baron d’Holbach’s Système de la Nature (1770) and David Hume’s Dialogues Concerning Natural Religion (1779) as well as the works of the French philosophers, Priestley maintained that materialism and determinism could be reconciled with a belief in God. He criticised those whose faith was shaped by books and fashion, drawing an analogy between the scepticism of educated men and the credulity of the masses.
Maintaining that humans had no free will, Priestley argued that what he called “philosophical necessity” (akin to absolute determinism) is consonant with Christianity, a position based on his understanding of the natural world. Like the rest of nature, man’s mind is subject to the laws of causation, Priestley contended, but because a benevolent God created these laws, the world and the people in it will eventually be perfected. Evil is therefore only an imperfect understanding of the world.
Although Priestley’s philosophical work has been characterised as “audacious and original”, it partakes of older philosophical traditions on the problems of free will, determinism, and materialism. For example, the 17th-century philosopher Baruch Spinoza argued for absolute determinism and absolute materialism. Like Spinoza and Priestley, Leibniz argued that human will was completely determined by natural laws; unlike them, Leibniz argued for a “parallel universe” of immaterial objects (such as human souls) so arranged by God that its outcomes agree exactly with those of the material universe. Leibniz and Priestley share an optimism that God has chosen the chain of events benevolently; however, Priestley believed that the events were leading to a glorious millennial conclusion, whereas for Leibniz the entire chain of events was optimal in and of itself, as compared with other conceivable chains of events.
Founder of British Unitarianism
When Priestley’s friend Theophilus Lindsey decided to found a new Christian denomination that would not restrict its members’ beliefs, Priestley and others hurried to his aid. On 17 April 1774, Lindsey held the first Unitarian service in Britain, at the newly formed Essex Street Chapel in London; he had even designed his own liturgy, of which many were critical. Priestley defended his friend in the pamphlet Letter to a Layman, on the Subject of the Rev. Mr. Lindsey’s Proposal for a Reformed English Church (1774), claiming that only the form of worship had been altered, not its substance, and attacking those who followed religion as a fashion. Priestley attended Lindsey’s church regularly in the 1770s and occasionally preached there. He continued to support institutionalised Unitarianism for the rest of his life, writing several Defenses of Unitarianism and encouraging the foundation of new Unitarian chapels throughout Britain and the United States.
Experiments and Observations on Different Kinds of Air
Priestley’s years in Calne were the only ones in his life dominated by scientific investigations; they were also the most scientifically fruitful. His experiments were almost entirely confined to “airs”, and out of this work emerged his most important scientific texts: the six volumes of Experiments and Observations on Different Kinds of Air (1774–86). These experiments helped repudiate the last vestiges of the theory of four elements, which Priestley attempted to replace with his own variation of phlogiston theory. According to that 18th-century theory, the combustion or oxidation of a substance corresponded to the release of a material substance, phlogiston.
Priestley’s work on “airs” is not easily classified. As historian of science Simon Schaffer writes, it “has been seen as a branch of physics, or chemistry, or natural philosophy, or some highly idiosyncratic version of Priestley’s own invention”. Furthermore, the volumes were both a scientific and a political enterprise for Priestley, in which he argues that science could destroy “undue and usurped authority” and that government has “reason to tremble even at an air pump or an electrical machine”.
Volume I of Experiments and Observations on Different Kinds of Air outlined several discoveries: “nitrous air” (nitric oxide, NO); “vapor of spirit of salt”, later called “acid air” or “marine acid air” (anhydrous hydrochloric acid, HCl); “alkaline air” (ammonia, NH3); “diminished” or “dephlogisticated nitrous air” (nitrous oxide, N2O); and, most famously, “dephlogisticated air” (oxygen, O2) as well as experimental findings that showed plants revitalised enclosed volumes of air, a discovery that would eventually lead to the discovery of photosynthesis. Priestley also developed a “nitrous air test” to determine the “goodness of air”. Using a pneumatic trough, he would mix nitrous air with a test sample, over water or mercury, and measure the decrease in volume—the principle of eudiometry. After a small history of the study of airs, he explained his own experiments in an open and sincere style. As an early biographer writes, “whatever he knows or thinks he tells: doubts, perplexities, blunders are set down with the most refreshing candour.” Priestley also described his cheap and easy-to-assemble experimental apparatus; his colleagues therefore believed that they could easily reproduce his experiments. Faced with inconsistent experimental results, Priestley employed phlogiston theory. This led him to conclude that there were only three types of “air”: “fixed”, “alkaline”, and “acid”. Priestley dismissed the burgeoning chemistry of his day. Instead, he focused on gases and “changes in their sensible properties”, as had natural philosophers before him. He isolated carbon monoxide (CO), but apparently did not realise that it was a separate “air”.
Discovery of oxygen
In August 1774 he isolated an “air” that appeared to be completely new, but he did not have an opportunity to pursue the matter because he was about to tour Europe with Shelburne. While in Paris, Priestley replicated the experiment for others, including French chemist Antoine Lavoisier. After returning to Britain in January 1775, he continued his experiments and discovered “vitriolic acid air” (sulphur dioxide, SO2).
In March he wrote to several people regarding the new “air” that he had discovered in August. One of these letters was read aloud to the Royal Society, and a paper outlining the discovery, titled “An Account of further Discoveries in Air”, was published in the Society’s journal Philosophical Transactions. Priestley called the new substance “dephlogisticated air”, which he made in the famous experiment by focusing the sun’s rays on a sample of mercuric oxide. He first tested it on mice, who surprised him by surviving quite a while entrapped with the air, and then on himself, writing that it was “five or six times better than common air for the purpose of respiration, inflammation, and, I believe, every other use of common atmospherical air”. He had discovered oxygen gas (O2).
Priestley assembled his oxygen paper and several others into a second volume of Experiments and Observations on Air, published in 1776. He did not emphasise his discovery of “dephlogisticated air” (leaving it to Part III of the volume) but instead argued in the preface how important such discoveries were to rational religion. His paper narrated the discovery chronologically, relating the long delays between experiments and his initial puzzlements; thus, it is difficult to determine when exactly Priestley “discovered” oxygen. Such dating is significant as both Lavoisier and Swedish pharmacist Carl Wilhelm Scheele have strong claims to the discovery of oxygen as well, Scheele having been the first to isolate the gas (although he published after Priestley) and Lavoisier having been the first to describe it as purified “air itself entire without alteration” (that is, the first to explain oxygen without phlogiston theory).
In his paper “Observations on Respiration and the Use of the Blood”, Priestley was the first to suggest a connection between blood and air, although he did so using phlogiston theory. In typical Priestley fashion, he prefaced the paper with a history of the study of respiration. A year later, clearly influenced by Priestley, Lavoisier was also discussing respiration at the Académie des sciences. Lavoisier’s work began the long train of discovery that produced papers on oxygen respiration and culminated in the overthrow of phlogiston theory and the establishment of modern chemistry.
Around 1779 Priestley and Shelburne – soon to be the 1st Marquess of Landsdowne – had a rupture, the precise reasons for which remain unclear. Shelburne blamed Priestley’s health, while Priestley claimed Shelburne had no further use for him. Some contemporaries speculated that Priestley’s outspokenness had hurt Shelburne’s political career. Schofield argues that the most likely reason was Shelburne’s recent marriage to Louisa Fitzpatrick—apparently, she did not like the Priestleys. Although Priestley considered moving to America, he eventually accepted Birmingham New Meeting’s offer to be their minister.
Both Priestley and Shelburne’s families upheld their Unitarian faith for generations. In December 2013, it was reported that Sir Christopher Bullock – a direct descendant of Shelburne’s brother, Thomas Fitzmaurice (MP) – had married his wife, Lady Bullock, née Barbara May Lupton, at London’s Unitarian Essex Church in 1917. Barbara Lupton was the second cousin of Olive Middleton, née Lupton, the great-grandmother of Catherine, Duchess of Cambridge. In 1914, Olive and Noel Middleton had married at Leeds’ Mill Hill Chapel, which Priestley, as its minister, had once guided towards Unitarianism.
Birmingham (1780–1791)
In 1780 the Priestleys moved to Birmingham and spent a happy decade surrounded by old friends, until they were forced to flee in 1791 by religiously motivated mob violence in what became known as the Priestley Riots. Priestley accepted the ministerial position at New Meeting on the condition that he be required to preach and teach only on Sundays, so that he would have time for his writing and scientific experiments. As in Leeds, Priestley established classes for the youth of his parish and by 1781, he was teaching 150 students. Because Priestley’s New Meeting salary was only 100 guineas, friends and patrons donated money and goods to help continue his investigations. He was elected a Foreign Honorary Member of the American Academy of Arts and Sciences in 1782.
Chemical Revolution
Many of the friends that Priestley made in Birmingham were members of the Lunar Society, a group of manufacturers, inventors, and natural philosophers who assembled monthly to discuss their work. The core of the group included men such as the manufacturer Matthew Boulton, the chemist and geologist James Keir, the inventor and engineer James Watt, and the botanist, chemist, and geologist William Withering. Priestley was asked to join this unique society and contributed much to the work of its members. As a result of this stimulating intellectual environment, he published several important scientific papers, including “Experiments relating to Phlogiston, and the seeming Conversion of Water into Air” (1783). The first part attempts to refute Lavoisier’s challenges to his work on oxygen; the second part describes how steam is “converted” into air. After several variations of the experiment, with different substances as fuel and several different collecting apparatuses (which produced different results), he concluded that air could travel through more substances than previously surmised, a conclusion “contrary to all the known principles of hydrostatics”. This discovery, along with his earlier work on what would later be recognised as gaseous diffusion, would eventually lead John Dalton and Thomas Graham to formulate the kinetic theory of gases.
In 1777, Antoine Lavoisier had written Mémoire sur la combustion en général, the first of what proved to be a series of attacks on phlogiston theory; it was against these attacks that Priestley responded in 1783. While Priestley accepted parts of Lavoisier’s theory, he was unprepared to assent to the major revolutions Lavoisier proposed: the overthrow of phlogiston, a chemistry based conceptually on elements and compounds, and a new chemical nomenclature. Priestley’s original experiments on “dephlogisticated air” (oxygen), combustion, and water provided Lavoisier with the data he needed to construct much of his system; yet Priestley never accepted Lavoisier’s new theories and continued to defend phlogiston theory for the rest of his life. Lavoisier’s system was based largely on the quantitative concept that mass is neither created nor destroyed in chemical reactions (i.e., the conservation of mass). By contrast, Priestley preferred to observe qualitative changes in heat, color, and particularly volume. His experiments tested “airs” for “their solubility in water, their power of supporting or extinguishing flame, whether they were respirable, how they behaved with acid and alkaline air, and with nitric oxide and inflammable air, and lastly how they were affected by the electric spark.”
By 1789, when Lavoisier published his Traité Élémentaire de Chimie and founded the Annales de Chimie, the new chemistry had come into its own. Priestley published several more scientific papers in Birmingham, the majority attempting to refute Lavoisier. Priestley and other Lunar Society members argued that the new French system was too expensive, too difficult to test, and unnecessarily complex. Priestley in particular rejected its “establishment” aura. In the end, Lavoisier’s view prevailed: his new chemistry introduced many of the principles on which modern chemistry is founded.
Priestley’s refusal to accept Lavoisier’s “new chemistry”—such as the conservation of mass—and his determination to adhere to a less satisfactory theory has perplexed many scholars. Schofield explains it thus: “Priestley was never a chemist; in a modern, and even a Lavoisierian, sense, he was never a scientist. He was a natural philosopher, concerned with the economy of nature and obsessed with an idea of unity, in theology and in nature.” Historian of science John McEvoy largely agrees, writing that Priestley’s view of nature as coextensive with God and thus infinite, which encouraged him to focus on facts over hypotheses and theories, prompted him to reject Lavoisier’s system. McEvoy argues that “Priestley’s isolated and lonely opposition to the oxygen theory was a measure of his passionate concern for the principles of intellectual freedom, epistemic equality and critical inquiry.” Priestley himself claimed in the last volume of Experiments and Observations that his most valuable works were his theological ones because they were “superior [in] dignity and importance”.
Defender of English Dissenters and French revolutionaries
Although Priestley was busy defending phlogiston theory from the “new chemists”, most of what he published in Birmingham was theological. In 1782 he published the fourth volume of his Institutes, An History of the Corruptions of Christianity, describing how he thought the teachings of the early Christian church had been “corrupted” or distorted. Schofield describes the work as “derivative, disorganized, wordy, and repetitive, detailed, exhaustive, and devastatingly argued”. The text addresses issues ranging from the divinity of Christ to the proper form for the Lord’s Supper. Priestley followed up in 1786 with the provocatively titled book, An History of Early Opinions concerning Jesus Christ, compiled from Original Writers, proving that the Christian Church was at first Unitarian. Thomas Jefferson would later write of the profound effect that these two books had on him: “I have read his Corruptions of Christianity, and Early Opinions of Jesus, over and over again; and I rest on them … as the basis of my own faith. These writings have never been answered.” Although a few readers such as Jefferson and other Rational Dissenters approved of the work, it was harshly reviewed because of its extreme theological positions, particularly its rejection of the Trinity.
In 1785, while Priestley was engaged in a pamphlet war over Corruptions, he also published The Importance and Extent of Free Enquiry, claiming that the Reformation had not really reformed the church. In words that would boil over into a national debate, he challenged his readers to enact change:
Let us not, therefore, be discouraged, though, for the present, we should see no great number of churches professedly unitarian …. We are, as it were, laying gunpowder, grain by grain, under the old building of error and superstition, which a single spark may hereafter inflame, so as to produce an instantaneous explosion; in consequence of which that edifice, the erection of which has been the work of ages, may be overturned in a moment, and so effectually as that the same foundation can never be built upon again ….
Although discouraged by friends from using such inflammatory language, Priestley refused to back down from his opinions in print and he included it, forever branding himself as “Gunpowder Joe”. After the publication of this seeming call for revolution in the midst of the French Revolution, pamphleteers stepped up their attacks on Priestley and he and his church were even threatened with legal action.
In 1787, 1789, and 1790, Dissenters again tried to repeal the Test and Corporation Acts. Although initially it looked as if they might succeed, by 1790, with the fears of revolution looming in Parliament, few were swayed by appeals to equal rights. Political cartoons, one of the most effective and popular media of the time, skewered the Dissenters and Priestley. In Parliament, William Pitt and Edmund Burke argued against the repeal, a betrayal that angered Priestley and his friends, who had expected the two men’s support. Priestley wrote a series of Letters to William Pitt and Letters to Burke in an attempt to persuade them otherwise, but these publications only further inflamed the populace against him.
Dissenters such as Priestley who supported the French Revolution came under increasing suspicion as scepticism regarding the revolution grew. In its propaganda against “radicals”, Pitt’s administration used the “gunpowder” statement to argue that Priestley and other Dissenters wanted to overthrow the government. Burke, in his famous Reflections on the Revolution in France (1790), tied natural philosophers, and specifically Priestley, to the French Revolution, writing that radicals who supported science in Britain “considered man in their experiments no more than they do mice in an air pump”. Burke also associated republican principles with alchemy and insubstantial air, mocking the scientific work done by both Priestley and French chemists. He made much in his later writings of the connections between “Gunpowder Joe”, science, and Lavoisier—who was improving gunpowder for the French in their war against Britain. Paradoxically, a secular statesman, Burke, argued against science and maintained that religion should be the basis of civil society, whereas a Dissenting minister, Priestley, argued that religion could not provide the basis for civil society and should be restricted to one’s private life.
Priestley also supported the campaign to abolish the British slave trade, and published a sermon in 1788 in which he declared that nobody treated enslaved people “with so much cruelty as the English”.
Birmingham riots of 1791
The animus that had been building against Dissenters and supporters of the American and French Revolutions exploded in July 1791. Priestley and several other Dissenters had arranged to have a celebratory dinner on the anniversary of the storming of the Bastille, a provocative action in a country where many disapproved of the French Revolution and feared that it might spread to Britain. Amid fears of violence, Priestley was convinced by his friends not to attend. Rioters gathered outside the hotel during the banquet and attacked the attendees as they left. The rioters moved on to the New Meeting and Old Meeting churches—and burned both to the ground. Priestley and his wife fled from their home; although their son William and others stayed behind to protect their property, the mob overcame them and torched Priestley’s house “Fairhill” at Sparkbrook, destroying his valuable laboratory and all of the family’s belongings. Twenty-six other Dissenters’ homes and three more churches were burned in the three-day riot. Priestley spent several days hiding with friends until he was able to travel safely to London. The carefully executed attacks of the “mob” and the farcical trials of only a handful of the “leaders” convinced many at the time—and modern historians later—that the attacks were planned and condoned by local Birmingham magistrates. When George III was eventually forced to send troops to the area, he said: “I cannot but feel better pleased that Priestley is the sufferer for the doctrines he and his party have instilled, and that the people see them in their true light.”
Hackney (1791–1794)
… Lo! Priestley there, patriot, and saint, and sage,
Him, full of years, from his loved native land
Statesmen blood-stained and priests idolatrous
By dark lies maddening the blind multitude
Drove with vain hate ….
From “Religious Musings” (1796) by Samuel Taylor Coleridge
Unable to return to Birmingham, the Priestleys eventually settled in Lower Clapton, a district in Hackney, Middlesex where he gave a series of lectures on history and natural philosophy at the Dissenting academy, the New College at Hackney. Friends helped the couple rebuild their lives, contributing money, books, and laboratory equipment. Priestley tried to obtain restitution from the government for the destruction of his Birmingham property, but he was never fully reimbursed. He also published An Appeal to the Public on the Subject of the Riots in Birmingham (1791), which indicted the people of Birmingham for allowing the riots to occur and for “violating the principles of English government”.
The couple’s friends urged them to leave Britain and emigrate to either France or the new United States, even though Priestley had received an appointment to preach for the Gravel Pit Meeting congregation. Priestley was minister between 1793 and 1794 and the sermons he preached there, particularly the two Fast Sermons, reflect his growing millenarianism, his belief that the end of the world was fast approaching. After comparing Biblical prophecies to recent history, Priestley concluded that the French Revolution was a harbinger of the Second Coming of Christ. Priestley’s works had always had a millennial cast, but after the beginning of the French Revolution, this strain increased. He wrote to a younger friend that while he himself would not see the Second Coming, his friend “may probably live to see it … It cannot, I think be more than twenty years [away].”
Daily life became more difficult for the family: Priestley was burned in effigy along with Thomas Paine; vicious political cartoons continued to be published about him; letters were sent to him from across the country, comparing him to the devil and Guy Fawkes; tradespeople feared the family’s business; and Priestley’s Royal Academy friends distanced themselves. As the penalties became harsher for those who spoke out against the government, Priestley examined options for removing himself and his family from England.[citation needed]
Joseph Priestley’s son William was presented to the French Assembly and granted letters of naturalisation on 8 June 1792. Priestley learned about it from the Morning Chronicle. A decree of 26 August 1792 by the French National Assembly conferred French citizenship on Joseph Priestley and others who had “served the cause of liberty” by their writings. Priestley accepted French citizenship, considering it “the greatest of honours”. In the French National Convention election on 5 September 1792, Joseph Priestley was elected to the French National Convention by at least two departments, (Orne and Rhône-et-Loire). He declined the honour, on the grounds that he was not fluent in French.
As relations between England and France worsened, a removal to France became impracticable. Following the declaration of war of February 1793, and the Aliens Bill of March 1793, which forbade correspondence or travel between England and France, William Priestley left France for America. Joseph Priestley’s sons Harry and Joseph chose to leave England for America in August 1793. Finally Priestley himself followed with his wife, boarding the Sansom at Gravesend on 7 April 1794. Five weeks after Priestley left, William Pitt’s administration began arresting radicals for seditious libel, resulting in the famous 1794 Treason Trials.
Pennsylvania (1794–1804)
The Priestleys arrived in New York City on 4 June 1794, where they were fêted by various political factions vying for Priestley’s endorsement. Priestley declined their entreaties, hoping to avoid political discord in his new country. Before travelling to a new home in the backwoods of Northumberland County, Pennsylvania, at Point township (now the Borough of Northumberland), Priestley and his wife lodged in Philadelphia, where he gave a series of sermons which led to the founding of the First Unitarian Church of Philadelphia. Priestley turned down an opportunity to teach chemistry at the University of Pennsylvania.
Priestley’s son Joseph Priestley Jr. was a leading member of a consortium that had purchased 300,000 acres (120,000 ha) of virgin woodland between the forks of Loyalsock Creek. This they intended to lease or sell in 400-acre (160 ha) plots, with payment deferred to seven annual instalments, with interest. His brothers, William and Henry, bought a 284-acre (115 ha) plot of woodland which they attempted to transform into a farm, later called “Fairhill”, felling and uprooting trees, and making lime to sweeten the soil by building their own lime kilns. Henry Priestley died 11 December 1795, possibly of malaria which he may have contracted after landing at New York. Mary Priestley’s health, already poor, deteriorated further; although William’s wife, Margaret Foulke-Priestley, moved in with the couple to nurse Mary 24 hours a day, Mary Priestley died 17 September 1796. Priestley then moved in with his elder son, Joseph Jr., and his wife Elizabeth Ryland-Priestley.[citation needed] Thomas Cooper, whose son, Thomas Jr., was living with the Priestleys, was a frequent visitor.[citation needed]
Since his arrival in America, Priestley had continued to defend his Christian Unitarian beliefs; now, falling increasingly under the influence of Thomas Cooper and Elizabeth Ryland-Priestley, he was unable to avoid becoming embroiled in political controversy. In 1798, when, in response to the Pinckney affair, a belligerent President Adams sought to enlarge the navy and mobilise the militia into what Priestley and Cooper saw as a ‘standing army’, Priestley published an anonymous newspaper article: Maxims of political arithmetic, which attacked Adams, defended free trade, and advocated a form of Jeffersonian isolationism. In the same year, a small package, addressed vaguely: “Dr Priestley in America,” was seized by the Royal Navy on board a neutral Danish boat. It was found to contain three letters, one of which was signed by the radical printer John Hurford Stone. These intercepted letters were published in London, and copied in numerous papers in America. One of the letters was addressed to “MBP”, with a note: “I inclose a note for our friend MBP—but, as ignorant of the name he bears at present among you, I must beg you to seal and address it.” This gave the intercepted letters a tinge of intrigue. Fearful lest they be taken as evidence of him being a ‘spy in the interest of France’, Priestley sent a clumsy letter to numerous newspaper editors, in which he naively named “MBP” (Member of the British Parliament) as Mr. Benjamin Vaughan, who “like me, thought it necessary to leave England, and for some time is said to have assumed a feigned name.” William Cobbett, in his Porcupine’s Gazette, 20 August 1798, added that Priestley “has told us who Mr MBP is, and has confirmed me in the opinion of their both being spies in the interest of France.”
Joseph Priestley Jr. left on a visit to England at Christmas 1798, not returning until August 1800. In his absence, his wife Elizabeth Ryland-Priestley and Thomas Cooper became increasing close, collaborating in numerous political essays. Priestley allowed himself to fall too heavily under Elizabeth and Cooper’s influences, even helping hawk a seditious handbill Cooper had printed, around Point township, and across the Susquehanna at Sunbury. In September 1799, William Cobbett printed extracts from this handbill, asserting that: “Dr Priestley has taken great pains to circulate this address, has travelled through the country for the purpose, and is in fact the patron of it.” He challenged Priestley to “clear himself of the accusation” or face prosecution. Barely a month later, in November and December 1799, Priestley stepped forward in his own defence, with his Letters to the inhabitants of Northumberland.
Priestley’s son, William, now living in Philadelphia, was increasingly embarrassed by his father’s actions. He confronted his father, expressing John and Benjamin Vaughan’s unease, his own wife’s concerns about Elizabeth Ryland-Priestley’s dietary care, and his own concerns at the closeness of Elizabeth Ryland-Priestley and Thomas Cooper’s relationship, and their adverse influence on Dr Priestley; but this only led to a further estrangement between William and his sister-in-law. When, a while later, Priestley’s household suffered a bout of food poisoning, perhaps from milk sickness or a bacterial infection, Elizabeth Ryland-Priestley falsely accused William of having poisoned the family’s flour. Although this allegation has attracted the attention of some modern historians, it is believed to be without foundation.
Priestley continued the educational projects that had always been important to him, helping to establish the “Northumberland Academy” and donating his library to the fledgling institution. He exchanged letters regarding the proper structure of a university with Thomas Jefferson, who used this advice when founding the University of Virginia. Jefferson and Priestley became close, and when the latter had completed his General History of the Christian Church, he dedicated it to President Jefferson, writing that “it is now only that I can say I see nothing to fear from the hand of power, the government under which I live being for the first time truly favourable to me.”
Priestley tried to continue his scientific investigations in America with the support of the American Philosophical Society, to which he had been previously elected a member in 1785. He was hampered by lack of news from Europe; unaware of the latest scientific developments, Priestley was no longer on the forefront of discovery. Although the majority of his publications focused on defending phlogiston theory, he also did some original work on spontaneous generation and dreams. Despite Priestley’s reduced scientific output, his presence stimulated American interest in chemistry.
By 1801, Priestley had become so ill that he could no longer write or experiment. He died on the morning of 6 February 1804, aged seventy and was buried at Riverview Cemetery in Northumberland, Pennsylvania.
Priestley’s epitaph reads:
Return unto thy rest, O my soul, for the
Lord hath dealt bountifully with thee.
I will lay me down in peace and sleep till
I awake in the morning of the resurrection.
Legacy
By the time he died in 1804, Priestley had been made a member of every major scientific society in the Western world and he had discovered numerous substances. The 19th-century French naturalist George Cuvier, in his eulogy of Priestley, praised his discoveries while at the same time lamenting his refusal to abandon phlogiston theory, calling him “the father of modern chemistry [who] never acknowledged his daughter”. Priestley published more than 150 works on topics ranging from political philosophy to education to theology to natural philosophy. He led and inspired British radicals during the 1790s, paved the way for utilitarianism, and helped found Unitarianism. A wide variety of philosophers, scientists, and poets became associationists as a result of his redaction of David Hartley’s Observations on Man, including Erasmus Darwin, Coleridge, William Wordsworth, John Stuart Mill, Alexander Bain, and Herbert Spencer. Immanuel Kant praised Priestley in his Critique of Pure Reason (1781), writing that he “knew how to combine his paradoxical teaching with the interests of religion”. Indeed, it was Priestley’s aim to “put the most ‘advanced’ Enlightenment ideas into the service of a rationalized though heterodox Christianity, under the guidance of the basic principles of scientific method”.
Considering the extent of Priestley’s influence, relatively little scholarship has been devoted to him. In the early 20th century, Priestley was most often described as a conservative and dogmatic scientist who was nevertheless a political and religious reformer. In a historiographic review essay, historian of science Simon Schaffer describes the two dominant portraits of Priestley: the first depicts him as “a playful innocent” who stumbled across his discoveries; the second portrays him as innocent as well as “warped” for not understanding their implications better. Assessing Priestley’s works as a whole has been difficult for scholars because of his wide-ranging interests. His scientific discoveries have usually been divorced from his theological and metaphysical publications to make an analysis of his life and writings easier, but this approach has been challenged recently by scholars such as John McEvoy and Robert Schofield. Although early Priestley scholarship claimed that his theological and metaphysical works were “distractions” and “obstacles” to his scientific work, scholarship published in the 1960s, 1970s, and 1980s maintained that Priestley’s works constituted a unified theory. However, as Schaffer explains, no convincing synthesis of his work has yet been expounded. More recently, in 2001, historian of science Dan Eshet has argued that efforts to create a “synoptic view” have resulted only in a rationalisation of the contradictions in Priestley’s thought, because they have been “organized around philosophical categories” and have “separate[d] the producers of scientific ideas from any social conflict”.
Priestley has been remembered by the towns in which he served as a reforming educator and minister and by the scientific organisations he influenced. Two educational institutions have been named in his honour—Priestley College in Warrington and Joseph Priestley College in Leeds (now part of Leeds City College)—and an asteroid, 5577 Priestley, discovered in 1986 by Duncan Waldron. In Birstall, the Leeds City Square, and in Birmingham, he is memorialised through statues, and plaques commemorating him have been posted in Birmingham, Calne and Warrington. The main undergraduate chemistry laboratories at the University of Leeds were refurbished as part of a £4m refurbishment plan in 2006 and renamed as the Priestley Laboratories in his honour as a prominent chemist from Leeds. In 2016 the University of Huddersfield renamed the building housing its Applied Sciences department as the Joseph Priestley Building, as part of an effort to rename all campus buildings after prominent local figures.
Since 1952 Dickinson College, Pennsylvania, has presented the Priestley Award to a “distinguished scientist whose work has contributed to the welfare of humanity”. Priestley’s work is recognised by a National Historic Chemical Landmark designation for his discovery of oxygen, made on 1 August 1994, at the Priestley House in Northumberland, Penn., by the American Chemical Society. Similar recognition was made on 7 August 2000, at Bowood House in Wiltshire, England. The ACS also awards their highest honour, the Priestley Medal, in his name.
Several of his descendants became physicians, including the noted American surgeon James Taggart Priestley II of the Mayo Clinic.
Archives
Papers of Joseph Priestley are held at the Cadbury Research Library, University of Birmingham.
Selected works
Library resources about |
| By Joseph Priestley |
|---|
- The Rudiments of English Grammar (1761)
- A Chart of Biography (1765)
- Essay on a Course of Liberal Education for Civil and Active Life (1765)
- The History and Present State of Electricity (1767)
- Essay on the First Principles of Government (1768)
- A New Chart of History (1769)
- Institutes of Natural and Revealed Religion (1772–74)
- Experiments and Observations on Different Kinds of Air (1774–77)
- Disquisitions Relating to Matter and Spirit (1777)
- The Doctrine of Philosophical Necessity Illustrated (1777)
- Letters to a Philosophical Unbeliever (1780)
- An History of the Corruptions of Christianity (1782)
- Lectures on History and General Policy (1788)
- Theological Repository (1770–73, 1784–88)
See also
- List of independent discoveries
- List of liberal theorists
- Timeline of hydrogen technologies
- Bibliography of Benjamin Franklin — Many works on Franklin make reference to Priestley
Citations
- “List of Fellows of the Royal Society 1660 – 2007, K – Z”. royalsociety.org. The Royal Society. Archived from the original on 12 December 2007. Retrieved 1 August 2019.
- “Copley archive winners 1799–1731”. royalsociety.org. The Royal Society. Archived from the original on 11 January 2008. Retrieved 1 August 2019.
- “Priestley” Archived 30 October 2014 at the Wayback Machine: Collins English Dictionary – Complete & Unabridged 2012 Digital Edition.
- Gray & Harrison: Oxford Dictionary of National Biography, pp. 351-352
- Isaacson, 2004, pp. 140–141, 289
- Schofield, 1997, p. 142
- H. I. Schlesinger (1950). General Chemistry (4th ed.). p. 134.
- Although Swedish chemist Carl Wilhelm Scheele also has strong claims to the discovery, Priestley published his findings first. Scheele discovered it by heating potassium nitrate, mercuric oxide, and many other substances in about 1772.
- “Joseph Priestley, Discoverer of Oxygen National Historic Chemical Landmark”. American Chemical Society. Retrieved 21 May 2021.
- Tapper, 10.
- Tapper, 314.
- Van Doren, p. 420
- Schofield, 1997, p. 274
- Schofield (1997), 2.
- Schofield (1997), 2–12; Uglow, 72; Jackson, 19–25; Gibbs, 1–4; Thorpe, 1–11; Holt, 1–6.
- Schofield (1997), 1, 7–8; Jackson, 25–30; Gibbs, 4; Priestley, Autobiography, 71–73, 123.
- Schofield (1997), 14, 28–29; Uglow, 72; Gibbs, 5; Thorpe, 11–12; Holt, 7–9.
- Schofield (1997), 28–29; Jackson, 30; Gibbs, 5.
- McEvoy (1983), 48–49.
- Qtd. in Jackson, 33. See Schofield (1997), 40–57; Uglow, 73–74; Jackson, 30–34; Gibbs, 5–10; Thorpe, 17–22; Tapper, 314; Holt, 11–14; Garrett, 54.
- Schofield (1997), 62–69.
- Schofield (1997), 62–69; Jackson, 44–47; Gibbs, 10–11; Thorpe, 22–29; Holt, 15–19.
- Priestley, Joseph. The Rudiments of English Grammar; adapted to the use of schools. With observations on style. London: Printed for R. Griffiths, 1761.
- Qtd. in Schofield (1997), 79.
- Schofield (1997), 77–79, 83–85; Uglow, 72; Jackson 49–52; Gibbs, 13–16; Thorpe, 30–32; Holt, 19–23.
- McLachlan, Iconography, 24–26.
- Schofield, Robert E. (2009). Enlightened joseph priestley : a study of his life and work from 1773 to 1804. University Park: Penn State Univ Press. ISBN 978-0-271-03625-0. Retrieved 26 June 2018.
- Meyer, Michal (2018). “Old Friends”. Distillations. 4 (1): 6–9. Retrieved 26 June 2018.
- Bowden, Mary Ellen; Rosner, Lisa, eds. (2005). Joseph Priestley, radical thinker : a catalogue to accompany the exhibit at the Chemical Heritage Foundation commemorating the 200th anniversary of the death of Joseph Priestley, 23 August 2004 to 29 July 2005. Philadelphia, Penns.: Chemical Heritage Foundation. p. 26. ISBN 978-0941901383. Archived from the original on 2 June 2016. Retrieved 11 September 2014.
- Priestley, Autobiography, 87.
- See Thorpe, 33–44 for a description of life at Warrington; Schofield (1997), 89–90, 93–94; Jackson, 54–58; Uglow, 73–75; Thorpe, 47–50; Holt, 27–28.
- Sheps, 135, 149; Holt, 29–30.
- Qtd. in Sheps, 146.
- Priestley, Joseph. Essay on a Course of Liberal Education for Civil and Active Life. London: Printed for C. Henderson under the Royal Exchange; T. Becket and De Hondt in the Strand; and by J. Johnson and Davenport, in Pater-Noster-Row, 1765.
- Thorpe, 52–54; Schofield (1997), 124–25; Watts, 89, 95–97; Sheps, 136.
- Schofield (1997), 121; see also Watts, 92.
- Schofield (2004), 254–59; McLachlan (1987–90), 255–58; Sheps, 138, 141; Kramnick, 12; Holt, 29–33.
- Priestley, Joseph. A Chart of Biography. London: J. Johnson, St. Paul’s Church Yard, 1765 and Joseph Priestley, A Description of a Chart of Biography. Warrington: Printed by William Eyres, 1765 and Joseph Priestley, A New Chart of History. London: Engraved and published for J. Johnson, 1769; A Description of a New Chart of History. London: Printed for J. Johnson, 1770.
- Rosenberg, 57–65 and ff.
- Gibbs, 37; Schofield (1997), 118–19.
- J. Priestley. A Course of Lectures on Oratory and Criticism. London, 1777. Ed. V. M. Bevilacqua & R. Murphy. Carbondale: Southern Illinois University Press, 1965.
- Schofield (1997), 136–37; Jackson, 57–61.
- Isaacson, 2004, pp. 140–141, 182
- Van Doren, pp. 164–165
- Schofield (1997), 141–42, 152; Jackson, 64; Uglow 75–77; Thorpe, 61–65.
- Schofield (1997), 143–44; Jackson, 65–66; see Schofield (1997), 152 and 231–32 for an analysis of the different editions.
- Priestley, Joseph. The History and Present State of Electricity, with original experiments. London: Printed for J. Dodsley, J. Johnson and T. Cadell, 1767.
- Schofield (1997), 144–56.
- Schofield (1997), 156–57; Gibbs 28–31; see also Thorpe, 64.
- Other early investigators who suspected that the electrical force diminished with distance as the gravitational force did (i.e., as the inverse square of the distance) included Daniel Bernoulli (see: Abel Socin (1760) Acta Helvetia, vol. 4, pp. 224–25.) and Alessandro Volta, both of whom measured the force between plates of a capacitor, and Aepinus. See: J.L. Heilbron, Electricity in the 17th and 18th Centuries: A Study of Early Modern Physics (Los Angeles, California: University of California Press, 1979), pp. 460–62, 464 Archived 14 May 2016 at the Wayback Machine (including footnote 44).
- Joseph Priestley, The History and Present State of Electricity, with Original Experiments (London, England: 1767), p. 732 Archived 28 May 2016 at the Wayback Machine:
May we not infer from this experiment, that the attraction of electricity is subject to the same laws with that of gravitation, and is therefore according to the squares of the distances; since it is easily demonstrated, that were the earth in the form of a shell, a body in the inside of it would not be attracted to one side more than another?
- Coulomb (1785) “Premier mémoire sur l’électricité et le magnétisme,” Histoire de l’Académie Royale des Sciences, pp. 569–577
- Priestley, Joseph. A Familiar Introduction to the Study of Electricity. London: Printed for J. Dodsley; T. Cadell; and J. Johnson, 1768.
- Schofield (1997), 228–30.
- Schofield (1997), 162–64.
- Priestley, Autobiography, 98; see also Schofield (1997), 163.
- Schofield (1997), 162, note 7.
- Schofield, (1997), 158, 164; Gibbs, 37; Uglow, 170.
- Schofield (1997), 165–69; Holt, 42–43.
- Schofield (1997), 170–71; Gibbs, 37; Watts, 93–94; Holt, 44.
- Priestley. Institutes of Natural and Revealed Religion. London: Printed for J. Johnson, Vol. I, 1772, Vol. II, 1773, Vol. III, 1774.
- Miller, xvi; Schofield (1997), 172.
- Schofield (1997), 174; Uglow, 169; Tapper, 315; Holt, 44.
- Qtd. in Jackson, 102.
- McLachlan (1987–90), 261; Gibbs, 38; Jackson, 102; Uglow, 169.
- Schofield (1997), 181.
- See Schofield (1997), 181–88 for analysis of these two controversies.
- See Schofield (1997), 193–201 for an analysis of the journal; Uglow, 169; Holt, 53–55.
- See Schofield (2004), 202–7 for an analysis of Priestley’s contributions.
- Schofield (1997), 207.
- Schofield (1997), 202–05; Holt, 56–64.
- Priestley, Joseph. Essay on the First Principles of Government; and on the nature of political, civil, and religious liberty. London: Printed for J. Dodsley; T. Cadell; and J. Johnson, 1768.
- Gibbs, 39–43; Uglow, 169; Garrett, 17; Tapper, 315; Holt, 34–37; Philip (1985); Miller, xiv.
- Priestley, Joseph. Remarks on some paragraphs in the fourth volume of Dr. Blackstone’s Commentaries on the laws of England, relating to the Dissenters. London: Printed for J. Johnson and J. Payne, 1769.
- Schofield (1997), 214–16; Gibbs, 43; Holt, 48–49.
- Qtd. in Kramnick, 8.
- Kramnick, 1981, p. 10
- Schofield (1997), 227, 232–38; see also Gibbs, 47; Kramnick, 9–10.
- Priestley, Joseph. Proposals for printing by subscription, The history and present state of discoveries relating to vision, light, and colours. Leeds: n.p., 1771.
- Moura, Breno (2018). “Newtonian Optics and the Historiography of Light in the 18th Century: A critical Analysis of Joseph Priestley’s The History of Optics”. Transversal: International Journal for the Historiography of Science (5). doi:10.24117/2526-2270.2018.i5.12. ISSN 2526-2270. S2CID 239593348.
- Schofield (1997), 240–49; Gibbs, 50–55; Uglow, 134.
- Priestley, Joseph. Directions for impregnating water with fixed air; in order to communicate to it the peculiar spirit and virtues of Pyrmont water, and other mineral waters of a similar nature. London: Printed for J. Johnson, 1772.
- Schofield (1997), 256–57; Gibbs, 57–59; Thorpe, 76–79; Uglow, 134–36; 232–34.
- Schils, René (2011). How James Watt Invented the Copier: Forgotten Inventions of Our Great Scientists. Springer Science & Business Media. p. 36.
- LaMoreaux, Philip E. (2012). Springs and Bottled Waters of the World: Ancient History, Source, Occurrence, Quality and Use. Springer Science & Business Media. p. 135.
- Schofield (1997), 251–55; see Holt, 64; Gibbs, 55–56; and Thorpe, 80–81, for the traditional account of this story.
- Schofield (1997), 270–71; Jackson, 120–22; Gibbs, 84–86: Uglow, 239–40; Holt, 64–65.
- McLachlan, Iconography, 19–20.
- Qtd. in Gibbs, 91.
- Schofield (2004), 4–11; 406; Gibbs, 91–94; Jackson, 122, 124, 143–52, 158–62; Thorpe, 80–85; Watts, 96; Holt, 70–94 (includes large quotations from Priestley’s letters sent from Europe to Shelburne’s sons).
- McEvoy and McGuire, 326–27; Tapper, 316.
- Schofield (2004), 72.
- Schofield (2004), 59–76; Gibbs, 99–100; Holt, 112–24; McEvoy and McGuire, 333–34.
- Tapper, 320; Priestley, Autobiography, 111; Schofield (2004), 37–42; Holt, 93–94; 139–42.
- Schofield (2004), 77–91; Garrett, 55; Tapper, 319; Sheps, 138; McEvoy (1983), 50; McEvoy and McGuire, 338–40.
- Sheps, 138.
- McEvoy and McGuire, 341–45.
- Leibniz, Gottfried Wilhelm. Confessio Philosophi: Papers Concerning the Problem of Evil, 1671–1678. Trans. Robert C. Sleigh, Jr. New Haven: Yale University Press (2004), xxxviii, 109. ISBN 978-0-300-08958-5. The original Latin text and an English translation of Leibniz’s A Philosopher’s Creed can be found on the Latin and English Wikisources, respectively.
- Stewart, Matthew. The Courtier and the Heretic: Leibniz, Spinoza, and the Fate of God in the Modern World. New York: W. W. Norton (2006), 171. ISBN 0-393-05898-0.
- McEvoy and McGuire, 341.
- Adams, Robert Merrihew. Leibniz: Determinist, Theist, Idealist. New York: Oxford University Press (1998), 10–13, 1–20, 41–44. ISBN 0-19-508460-8.
- Rutherford, 213–18.
- Rutherford, 46.
- Schofield (2004), 78–79.
- Rutherford, 12–15, 22–45, 49–54.
- Priestley, Joseph. Letter to a Layman, on the Subject of the Rev. Mr. Lindsey’s Proposal for a Reformed English Church. London: Printed for J. Wilkie, 1774.
- Schofield (2004), 26–28; Jackson, 124; Gibbs, 88–89; Holt, 56–64.
- Schofield (2004), 225, 236–38.
- Priestley, Joseph. Experiments and Observations on Different Kinds of Air. 3 vols. London W. Bowyer and J. Nichols, 1774–77. There are several different editions of these volumes, each important.
- See Gibbs 67–83 for a description of all of Priestley’s experiments during this time; Thorpe, 170ff.
- Thorpe, 167–68; Schofield (2004), 98–101.
- Schaffer, 152.
- Qtd. in Kramnick, 11–12; see also Schofield (2004), 121–24.
- Fruton, 20, 29
- Schofield (2004), 98; Thorpe, 171.
- Schofield (1997), 259–69; Jackson, 110–14; Thorpe, 76–77, 178–79; Uglow, 229–39.
- Schofield (2004), 93–105; Uglow, 240–41; see Gibbs 105–16 for a description of these experiments.
- Priestley, Joseph. “An Account of Further Discoveries in Air”. Philosophical Transactions 65 (1775): 384–94.
- Qtd. in Schofield (2004), 107.
- Wagner, P. (2012). Hypoxia. Springer. p. 10. ISBN 978-1-4419-8997-0. Retrieved 2 January 2024.
- Schofield (2004), 105–19; see also Jackson, 126–27, 163–64, 166–74; Gibbs, 118–23; Uglow, 229–31, 241; Holt, 93.
- Kuhn, 53–55.
- Schofield (2004), 129–30; Gibbs, 124–25.
- Schofield (2004), 141–43; see also Jackson, 198–99; Holt, 81–82.
- Nikkah, Roya (16 December 2012). “The Duchess discovers blue blood in her own family”. UK Sunday Telegraph. p. 9. Archived from the original on 29 October 2014. Retrieved 8 July 2014.
- Schofield (2004), 147–50, 196–99, 242–46. Gibbs, 134–40, 169; Uglow, 310–20, 407; Jackson, 227–28; Holt, 132–33.
- “Book of Members, 1780–2010: Chapter P” (PDF). American Academy of Arts and Sciences. Archived (PDF) from the original on 15 May 2011. Retrieved 28 July 2014.
- Schofield (2004), 151–52; for an analysis of Priestley’s contributions to each man’s work, see Schofield’s chapter “Science and the Lunar Society”; see also Jackson, 200–01; Gibbs, 141–47; Thorpe, 93–102; Holt, 127–32; Uglow, 349–50; for a history of the Lunar Society, see Uglow.
- Qtd. in Schofield (2004), 167
- Schofield (2004), 168; see also Jackson 203–08; Gibbs, 154–61; Uglow, 358–61.
- Memoirs of the Royal Academy of Sciences of Paris année 1777 (1780): 592–600. The next, most notable installment was “Réflexions sur le phlogistique, pour servir de suite à la théorie de la combustion et de la calcination publiée en 1777” Memoirs of the Royal Academy of Sciences of Paris année 1783 (1786): 505–538 (translated by Nicholas W. Best as “Lavoisier’s ‘Reflections on Phlogiston’ I: Against Phlogiston Theory”, Foundations of Chemistry 17 (2015): 137–151).
- Thorpe, 210; see also Schofield (2004), 169–94; Jackson 216–24.
- Schaffer, 164; Uglow, 356; McEvoy (1983), 56–57; Donovan, 175–76, 180–81.
- See Schaffer, 162–70 for a historiographical analysis.
- Schofield (2004), 194.
- McEvoy (1983), 51ff.
- McEvoy (1983), 57; see also McEvoy and MeGuire 395ff.
- Qtd. in Thorpe, 213.
- Priestley, Joseph. An History of the Corruptions of Christianity. 2 vols. Birmingham: Printed by Piercy and Jones; London: Printed for J. Johnson, 1782.
- Schofield (2004), 216.
- Qtd. in Gibbs, 249.
- Schofield (2004), 216–23; Thorpe, 106–08; Holt, 133–39; Philip (1985).
- Priestley, Joseph. The importance and extent of free inquiry in matters of religion: a sermon, preached before the congregations of the Old and New Meeting of Protestant Dissenters at Birmingham. 5 November 1785. To which are added, reflections on the present state of free inquiry in this country. Birmingham: Printed by M. Swinney; for J. Johnson, London, 1785.
- Qtd. in Gibbs, 173.
- Gibbs, 169–76; Uglow, 408.
- Gibbs, 176–83.
- Priestley, Joseph. A letter to the Right Honourable William Pitt, … on the subjects of toleration and church establishments; occasioned by his speech against the repeal of the Test and Corporation Acts, on Wednesday 28 March 1787. London: Printed for J. Johnson and J. Debrett, 1787.
- Priestley, Joseph. Letters to the Right Honourable Edmund Burke, occasioned by his Reflections on the Revolution in France, &c. Birmingham: Printed by Thomas Pearson; sold by J. Johnson, London, 1791.
- Schofield (2004), 269–81; Thorpe, 122–25; Uglow, 409, 435–38; Holt, 142ff; Philip (1985).
- Qtd. in Crossland, 294.
- Crossland, 283–87, 305.
- Kramnick, 22.
- Page, Anthony (2011). “Rational dissent, enlightenment and abolition of the British slave trade”. The Historical Journal. 54 (3): 748–49. doi:10.1017/S0018246X11000227. S2CID 145068908.
- Dionisio, Jennifer (Summer 2010). “Birmingham Toast”. Chemical Heritage Magazine. 28 (2): 18.
- Qtd. in Gibbs, 204; Schofield (2004), 264, 285, 289; Thorpe, 122–44; Uglow, 440–46; Jackson, 248–60; Rose, 68–88; Holt, 154ff.
- Coleridge, Samuel Taylor. “Religious Musings: A Desultory Poem, Written on the Christmas Eve of 1794”. Archived from the original on 12 January 2010. Retrieved 1 January 2010.
- “Joseph Priestley at hackney.gov.uk”. Archived from the original on 3 March 2016. Retrieved 11 June 2010.
- Schaffer, 160; Schofield (2004), 298–99; Thorpe, 145–46; Uglow, 446–49; Jackson, 300–05.
- Priestley, Joseph. An Appeal to the Public on the Subject of the Riots in Birmingham. To which are added, strictures on a pamphlet, entitled ‘Thoughts on the late riot at Birmingham.‘ Birmingham: Printed by J. Thompson; sold by J. Johnson, London, 1791.
- Qtd. in Schofield (2004), 295.
- A blue plaque marks the site of the Gravel Pit Meeting at Ram Place and a brown plaque the site of the Priestleys’ house at 113, Lower Clapton Road: Joseph Priestley at hackney.gov.uk Archived 3 March 2016 at the Wayback Machine
- Garrett, 53, 57, 61.
- Qtd. in Garrett, 62.
- Schofield (2004), p. 318.
- Gibbs (1965), p. 214.
- Gibbs (1965), p. 216; Schofield (2004), p. 318.
- Graham (1995), p. 26.
- Gibbs (1965), p. 216; Schofield,(2004), p. 318.
- Schwartz, A. Truman; McEvoy, John G., eds. (1990). Motion toward perfection : the achievement of Joseph Priestley. Boston, Mass.: Skinner House Books. p. 199. ISBN 978-1558960107. Archived from the original on 3 June 2016. Retrieved 11 September 2014.
- Graham (1995), p. 27; Schofield (2004), p. 318.
- Graham (1995), p. 33.
- Graham (1995), p. 35.
- Gibbs, 207–22; Schofield (2004), 304–18; Thorpe, 145–55; Uglow, 446–49, 453–54; Jackson, 300–05; Holt, 177–78.
- Schofield (2004), 329–30.
- Schofield (2004), 324–32; Thorpe, 155–57; Jackson, 310–14; Holt, 179ff.
- Mary Cathryne Park, Joseph Priestley and the problem of Pantisocrasy (Philadelphia, 1947), 14–24, 52–57. Penn State University Library, The Joseph Priestley Collection. “The Joseph Priestley Collection”. Archived from the original on 24 June 2013. Retrieved 21 June 2013. Property inventory assets and debts account book, 1807–1810
- Tony Rail, “William Priestley vindicated,” Enlightenment and Dissent no.28 (2012); 150–195. “Dr Williams’s Centre for Dissenting Studies”. Archived from the original on 24 December 2013. Retrieved 23 December 2013.
- Tony Rail, op. cit. 161.
- Rutt, I(ii), 354.
- McLachlan (1983), 34.
- Schofield (2004), 326.
- Signed ‘A Quaker in politics,’ the Maxims were printed over two days in the Aurora General Advertiser, 26 & 27 February 1798, and reprinted in both the Auroraand Carey’s United States’ Recorder, 31 March & 1 April 1799. See Rutt, XXV, 175-82.
- Copies of original letters recently written by persons in Paris to Dr. Priestley in America, taken on board of a neutral vessel (London, 1798). Federal Gazette (Baltimore, MD), 27 August 1798.
- Vaughan had fled to France in May 1794, when John Hurford Stone’s brother, William, was arrested and found to have a letter from Vaughan. In France, to avoid arrest as an Englishmen, he assumed the name of Jean Martin, and lived quietly at Passy. (John G. Alger, Englishmen in the French Revolution (London, 1889), 93).
- Tony Rail, op. cit.; Schofield (2004), 329–38; Gibbs, 234–37; Jackson, 317–18; Garrett, 63; Holt, 199–204.
- In December 1799, two of Elizabeth Ryland-Priestley’s essays, On the propriety and expediency of unlimited enquiry, and A Reply to [Thomas Cooper’s] Observations on the Fast Day [Cooper had challenged the power of a President to declare a day of fasting and prayer], were published as part of Political essays (Northumberland, PA, 1799). [Eugene Volokh: “Elizabeth Ryland-Priestley, Early American author on free speech”; New York University Journal of Law & Liberty, 4(2) (2009), 382–5].
- Tony Rail, op. cit. 166–7.
- Published in two parts, Northumberland-town PA, 1799; printed by Andrew Kennedy who printed the Sunbury and Northumberland Gazette. A pirate edition seems to have been published at Albany NY for Samuel Campbell of New York. (Robert E Schofield, A scientific autobiography of Joseph Priestley (Cambridge, MS, 1966), 303).
- Dr Priestley suffered a bilious and bowel condition throughout his adult life, with episodes of severe diarrhoea, for which Margaret Foulke-Priestley seems to have suggested a diet that used maize flour (US Cornmeal), and excluded wheat flour. (Tony Rail, op. cit. 156, 161).
- Tony Rail, op. cit.
- Priestley, Joseph. A General History of the Christian Church. Northumberland: Printed for the author by Andrew Kennedy, 1803.
- Qtd. in Schofield (2004), 339–43.
- “APS Member History”. search.amphilsoc.org. Retrieved 16 December 2020.
- Schofield (2004), 352–72; Gibbs, 244–46.
- Schofield (2004), 400–01; Gibbs, 247–48; Thorpe, 162–65; Jackson, 324–25; Holt, 213–16.
- In accordance with known birth-death dates. His original headstone gives his age as “LXXI” (71).
- For the original marker, see “Edgar Fahs Smith Collection”. Archived from the original on 23 December 2008. Retrieved 17 November 2006. See also page 153 of Walker, William H. (1927). “History of the Priestley house and the movement for its preservation”. Journal of Chemical Education. 4 (2): 150–158. Bibcode:1927JChEd…4..150W. doi:10.1021/ed004p150
- Qtd. in Schofield (2004), 401.
- Schofield (2004), 151–52.
- Qtd. in McLachlan (1987–90), 259–60.
- Thorpe, 74; Kramnick, 4.
- Tapper, 322.
- Schofield (2004), 3.
- Schofield (2004), 52–57; Holt, 111–12.
- McEvoy (1983), 47.
- Schaffer, 154–57.
- Eshet, 131.
- “Joseph Priestley College”. Joseph Priestley College. Archived from the original on 14 November 2007. Retrieved 1 January 2010.
- Schmadel, Lutz D. Dictionary of Minor Planet Names. 5th ed. Berlin and New York: Springer (2003), 474.
- The statue in Birmingham is a 1951 recast, in bronze, of a white marble original by Francis John Williamson, unveiled on 1 August 1874.
- The Lunar Society Moonstones honour Priestley in Birmingham. There are Blue Plaques commemorating him on the side of the Church of St. Michael and St. Joseph, New Meeting House Lane, Birmingham (Birmingham Civic Society Archived 10 February 2008 at the Wayback Machine Retrieved 1 January 2010), and another on the Warrington Salvation Army Citadel, once the home of Priestley (British Crystallographic Association Archived 25 September 2006 at the Wayback Machine Retrieved 1 January 2010).
- “Minister opens £4m ‘state-of-the-art’ chemistry facilities at Leeds”, 20 October 2006, University of Leeds, Retrieved 25 November 2018.
- “University of Huddersfield”. www.hud.ac.uk. Archived from the original on 5 January 2017. Retrieved 8 February 2017.
- “Joseph Priestley Award”. Dickinson College. Retrieved 4 May 2021.
- “National Historic Chemical Landmarks”. Joseph Priestley and the Discovery of Oxygen. American Chemical Society. 2000. Archived from the original on 26 December 2015.
- Raber, Linda R. (7 April 2008). “85th Anniversary of the Priestley Medal”. Chemical & Engineering News. American Chemical Society. Retrieved 12 November 2018.
- “Priestley, James Taggart (1903–1979)”. Plarr’s Lives of the Fellows, Royal College of Surgeons of England.
- “UoB Calmview5: Search results”. calmview.bham.ac.uk. Retrieved 28 January 2021.
Bibliography
The most exhaustive biography of Priestley is Robert Schofield’s two-volume work; several older one-volume treatments exist: those of Gibbs, Holt and Thorpe. Graham and Smith focus on Priestley’s life in America and Uglow and Jackson both discuss Priestley’s life in the context of other developments in science.
- Gibbs, F. W. Joseph Priestley: Adventurer in Science and Champion of Truth. London: Thomas Nelson and Sons, 1965.
- Graham, Jenny. Revolutionary in Exile: The Emigration of Joseph Priestley to America, 1794–1804. Transactions of the American Philosophical Society 85 (1995). ISBN 0-87169-852-8.
- Holt, Anne (1970) [1931]. A life of Joseph Priestley. Westport, Conn., Greenwood Press. ISBN 978-0-8371-4240-1.
- Jackson, Joe. A World on Fire: A Heretic, an Aristocrat and the Race to Discover Oxygen. New York: Viking, 2005. ISBN 0-670-03434-7.
- Isaacson, Walter (2004). Benjamin Franklin : an American life. New York : Simon & Schuster. ISBN 978-0-6848-07614.
- Johnson, Steven. The Invention of Air: A Story of Science, Faith, Revolution, and the Birth of America. New York: Riverhead, 2008. ISBN 1-59448-852-5.
- Kramnick, Isaac (January 1986). “Eighteenth-Century Science and Radical Social Theory: The Case of Joseph Priestley’s Scientific Liberalism”. Journal of British Studies. Cambridge University Press on behalf of The North American Conference on British Studies. 25 (1): 1–30. doi:10.1086/385852. JSTOR 175609. S2CID 197667044.
- Schofield, Robert E. (1997). The enlightenment of Joseph Priestley : a study of his life and work from 1733 to 1773. University Park, Pa. : Pennsylvania State University Press. ISBN 978-0-2710-1662-7.
- Schofield, Robert E. (2004). The Enlightened Joseph Priestley: A Study of His Life and Work from 1773 to 1804. Penn State Press. ISBN 978-0-2710-3246-7.
- Smith, Edgar F. Priestley in America, 1794–1804. Philadelphia: P. Blakiston’s Son and Co., 1920.
- Tapper, Alan. “Joseph Priestley”. Dictionary of Literary Biography 252: British Philosophers 1500–1799. Eds. Philip B. Dematteis and Peter S. Fosl. Detroit: Gale Group, 2002.
- Thorpe, T. E. Joseph Priestley. London: J. M. Dent, 1906.
- Uglow, Jenny. The Lunar Men: Five Friends Whose Curiosity Changed the World. New York: Farrar, Straus and Giroux, 2002. ISBN 0-374-19440-8.
- Van Doren, Carl (1938). Benjamin Franklin. New York, Garden City Publishing Company.
Secondary materials
- Anderson, R. G. W. and Christopher Lawrence. Science, Medicine and Dissent: Joseph Priestley (1733–1804). London: Wellcome Trust, 1987. ISBN 0-901805-28-9.
- Bowers, J. D. Joseph Priestley and English Unitarianism in America. University Park: Pennsylvania State University Press, 2007. ISBN 0-271-02951-X.
- Braithwaite, Helen. Romanticism, Publishing and Dissent: Joseph Johnson and the Cause of Liberty. New York: Palgrave Macmillan, 2003. ISBN 0-333-98394-7.
- Conant, J. B., ed. “The Overthrow of the Phlogiston Theory: The Chemical Revolution of 1775–1789”. Harvard Case Histories in Experimental Science. Cambridge: Harvard University Press, 1950.
- Crook, R. E. A Bibliography of Joseph Priestley. London: Library Association, 1966.
- Crossland, Maurice. “The Image of Science as a Threat: Burke versus Priestley and the ‘Philosophic Revolution'”. British Journal for the History of Science 20 (1987): 277–307.
- Donovan, Arthur. Antoine Lavoisier: Science, Administration and Revolution. Cambridge: Cambridge University Press, 1996. ISBN 0-521-56218-X
- Eshet, Dan. “Rereading Priestley”. History of Science 39.2 (2001): 127–59.
- Fitzpatrick, Martin. “Joseph Priestley and the Cause of Universal Toleration”. The Price-Priestley Newsletter 1 (1977): 3–30.
- Garrett, Clarke. “Joseph Priestley, the Millennium, and the French Revolution”. Journal of the History of Ideas 34.1 (1973): 51–66.
- Fruton, Joseph S. Methods and Styles in the Development of Chemistry. Philadelphia: American Philosophical Society, 2002. ISBN 0-87169-245-7.
- Gray, Henry Colin; Harrison, Brian Howard (2004). Joseph Priestly. Vol. XLV. Oxford; New York : Oxford University Press: Oxford dictionary of national biography. pp. 351–359–.
- Kramnick, Isaac. “Eighteenth-Century Science and Radical Social Theory: The Case of Joseph Priestley’s Scientific Liberalism”. Journal of British Studies 25 (1986): 1–30.
- Kuhn, Thomas. The Structure of Scientific Revolutions. 3rd ed. Chicago: University of Chicago Press, 1996. ISBN 0-226-45808-3.
- Haakonssen, Knud, ed. Enlightenment and Religion: Rational Dissent in Eighteenth-Century Britain. Cambridge: Cambridge University Press, 1996. ISBN 0-521-56060-8.
- McCann, H. Chemistry Transformed: The Paradigmatic Shift from Phlogiston to Oxygen. Norwood: Alex Publishing, 1978. ISBN 0-89391-004-X.
- McEvoy, John G. “Joseph Priestley, ‘Aerial Philosopher’: Metaphysics and Methodology in Priestley’s Chemical Thought, from 1762 to 1781”. Ambix 25 (1978): 1–55, 93–116, 153–75; 26 (1979): 16–30.
- McEvoy, John G. “Enlightenment and Dissent in Science: Joseph Priestley and the Limits of Theoretical Reasoning”. Enlightenment and Dissent 2 (1983): 47–68.
- McEvoy, John G. “Priestley Responds to Lavoisier’s Nomenclature: Language, Liberty, and Chemistry in the English Enlightenment”. Lavoisier in European Context: Negotiating a New Language for Chemistry. Eds. Bernadette Bensaude-Vincent and Ferdinando Abbri. Canton, MA: Science History Publications, 1995. ISBN 0-88135-189-X.
- McEvoy, John G. and J.E. McGuire. “God and Nature: Priestley’s Way of Rational Dissent”. Historical Studies in the Physical Sciences 6 (1975): 325–404.
- McLachlan, John. Joseph Priestley Man of Science 1733–1804: An Iconography of a Great Yorkshireman. Braunton and Devon: Merlin Books, 1983. ISBN 0-86303-052-1.
- McLachlan, John. “Joseph Priestley and the Study of History”. Transactions of the Unitarian Historical Society 19 (1987–90): 252–63.
- Philip, Mark. “Rational Religion and Political Radicalism”. Enlightenment and Dissent 4 (1985): 35–46.
- Rose, R. B. “The Priestley Riots of 1791”. Past and Present 18 (1960): 68–88.
- Rosenberg, Daniel. Joseph Priestley and the Graphic Invention of Modern Time. Studies in Eighteenth Century Culture 36(1) (2007): pp. 55–103.
- Rutherford, Donald. Leibniz and the Rational Order of Nature. Cambridge: Cambridge University Press, 1995. ISBN 0-521-46155-3.
- Schaffer, Simon. “Priestley Questions: An Historiographic Survey”. History of Science 22.2 (1984): 151–83.
- Sheps, Arthur. “Joseph Priestley’s Time Charts: The Use and Teaching of History by Rational Dissent in late Eighteenth-Century England”. Lumen 18 (1999): 135–54.
- Watts, R. “Joseph Priestley and Education”. Enlightenment and Dissent 2 (1983): 83–100.
Primary materials
- Lindsay, Jack, ed. Autobiography of Joseph Priestley. Teaneck: Fairleigh Dickinson University Press, 1970. ISBN 0-8386-7831-9.
- Miller, Peter N., ed. Priestley: Political Writings. Cambridge: Cambridge University Press, 1993. ISBN 0-521-42561-1.
- Passmore, John A., ed. Priestley’s Writings on Philosophy, Science and Politics. New York: Collier Books, 1964.
- Rutt, John T., ed. Collected Theological and Miscellaneous Works of Joseph Priestley. Two vols. London: George Smallfield, 1832.
- Rutt, John T., ed. Life and Correspondence of Joseph Priestley. Two vols. London: George Smallfield, 1831.
- Schofield, Robert E., ed. A Scientific Autobiography of Joseph Priestley (1733–1804): Selected Scientific Correspondence. Cambridge: MIT Press, 1966.
Johann Heinrich Pott, a student of one of Stahl’s students, expanded the theory and attempted to make it much more understandable to a general audience. He compared phlogiston to light or fire, saying that all three were substances whose natures were widely understood but not easily defined. He thought that phlogiston should not be considered as a particle but as an essence that permeates substances, arguing that in a pound of any substance, one could not simply pick out the particles of phlogiston. Pott also observed the fact that when certain substances are burned they increase in mass instead of losing the mass of the phlogiston as it escapes; according to him, phlogiston was the basic fire principle and could not be obtained by itself. Flames were considered to be a mix of phlogiston and water, while a phlogiston-and-earthy mixture could not burn properly. Phlogiston permeates everything in the universe, it could be released as heat when combined with an acid. Pott proposed the following properties:
- The form of phlogiston consists of a circular movement around its axis.
- When homogeneous it cannot be consumed or dissipated in a fire.
- The reason it causes expansion in most bodies is unknown, but not accidental. It is proportional to the compactness of the texture of the bodies or to the intimacy of their constitution.
- The increase of weight during calcination is evident only after a long time, and is due either to the fact that the particles of the body become more compact, decrease the volume and hence increase the density as in the case of lead, or those little heavy particles of air become lodged in the substance as in the case of powdered zinc oxide.
- Air attracts the phlogiston of bodies.
- When set in motion, phlogiston is the chief active principle in nature of all inanimate bodies.
- It is the basis of colours.
- It is the principal agent in fermentation.
Pott’s formulations proposed little new theory; he merely supplied further details and rendered existing theory more approachable to the common man.
- White, John Henry (1973). The History of Phlogiston Theory. New York: AMS Press Inc. ISBN 978-0404069308.
- Wikipedia https://en.wikipedia.org/wiki/Phlogiston_theory
BIOGRAPHY
Johann Heinrich Pott (6 October 1692 – 29 March 1777) was a Prussian physician and chemist. He is considered a pioneer of pyrochemistry. He examined the elements bismuth and manganese apart from attempting improvements to glass and porcelain production.
Pott was born in Halberstadt, son of the royal councillor Johann Andreas Pott (1662–1729) and Dorothea Sophia daughter of Andreas Machenau. He studied at the cathedral school in Halberstadt and Francke’s pedagogium before studying theology at the University of Halle. He then shifted to study medicine and chemistry under Georg Ernst Stahl. In 1713 he studied assaying at Mansfield under mining master Lages. He spent two years along with two of his brothers as travelling evangelists for the Community of True Inspiration but he left the sect in 1715 and returned to study chemistry at Halle, receiving a doctorate in 1716 on sulfur under Friedrich Hoffmann. He worked as a physician in Halberstadt before moving to Berlin in 1720 and became a professor of chemistry at the Collegium Medico Chirurgicum in 1724. Like his mentor Stahl, he was a promoter of the phlogiston theory. He succeeded Caspar Neumann (1683–1737) as professor of pharmaceutical chemistry. In 1753 he attempted to get his son-in-law Ernst Gottfried Kurella into a professorship and clashed publicly with Johann Theodor Eller whose student Brandes took the position. Pott’s chemistry contributions included the use of borax and phosphorus beads in analysis. He examined graphite which he differentiated from the contemporary idea that it was lead. Pott established a porcelain factory in Freienwalde under the orders of Frederick II. He examined the composition of pyrolusite.
Pott married the daughter of businessman Stanislaus Rücker.
PUBLICATIONS
- Exercitationes chymicae (1738)
- Collectiones observationum et animadversionum chymicarum, 1739 and 1741
- Lithogeognosia (1746–57)
REFERENCES
- Klein, Ursula (2004-11-05). “Not a Pure Science: Chemistry in the 18th and 19th Centuries”. Science. 306 (5698): 981–982. doi:10.1126/science.1089888. ISSN 0036-8075. S2CID 10923830.
- Cassebaum, Heinz (1979). “Die Stellung der Braunstein-Untersuchungen von J. H. Pott (1692-1777) in der Geschichte des Mangans”. Sudhoffs Archiv. 63 (2): 136–153. ISSN 0039-4564. JSTOR 20776589.
- Engel, Michael (2001). “Pott, Johann Heinrich”. Neue Deutsche Biographie (in German). Vol. 20. Berlin: Duncker & Humblot. p. 660.
- Hufbauer, Karl (1982). The Formation of the German Chemical Community, 1720-1795. University of California Press. pp. 176–177.
- Greenaway, Frank (1975). “Johann Heinrich Pott”. Dictionary of scientific biography. Vol. 11. p. 109.
- Wikipedia https://en.wikipedia.org/wiki/Johann_Heinrich_Pott
Johann Juncker also created a very complete picture of phlogiston. When reading Stahl’s work, he assumed that phlogiston was in fact very material. He, therefore, came to the conclusion that phlogiston has the property of levity, or that it makes the compound that it is in much lighter than it would be without the phlogiston. He also showed that air was needed for combustion by putting substances in a sealed flask and trying to burn them.
- White, John Henry (1973). The History of Phlogiston Theory. New York: AMS Press Inc. ISBN 978-0404069308.
Guillaume-François Rouelle brought the theory of phlogiston to France, and he was a very influential scientist and teacher so it gained quite a strong foothold very quickly. Many of his students became very influential scientists in their own right, Lavoisier included. The French viewed phlogiston as a very subtle principle that vanishes in all analysis, yet it is in all bodies. Essentially they followed straight from Stahl’s theory.
- White, John Henry (1973). The History of Phlogiston Theory. New York: AMS Press Inc. ISBN 978-0404069308.
- Leicester, Henry M.; Klickstein, Herbert S. (1965). A Source Book in Chemistry. Cambridge, Massachusetts: Harvard University Press.
Giovanni Antonio Giobert introduced Lavoisier’s work in Italy. Giobert won a prize competition from the Academy of Letters and Sciences of Mantua in 1792 for his work refuting phlogiston theory. He presented a paper at the Académie royale des Sciences of Turin on 18 March 1792, entitled Examen chimique de la doctrine du phlogistique et de la doctrine des pneumatistes par rapport à la nature de l’eau (“Chemical examination of the doctrine of phlogiston and the doctrine of pneumatists in relation to the nature of water”), which is considered the most original defence of Lavoisier’s theory of water composition to appear in Italy.
- Abbri, Ferdinando (2001). “GIOBERT, Giovanni Antonio”. Dizionario Biografico degli Italiani [Biographical Dictionary of the Italians]. 55. Retrieved 15 September 2017.
British chemist Elizabeth Fulhame demonstrated through experiment that many oxidation reactions occur only in the presence of water, that they directly involve water, and that water is regenerated and is detectable at the end of the reaction. Based on her experiments, she disagreed with some of the conclusions of Lavoisier as well as with the phlogiston theorists that he critiqued. Her book on the subject appeared in print soon after Lavoisier’s execution for Farm-General membership during the French Revolution.
- Rayner-Canham, Marelene; Rayner-Canham, Geoffrey (2001). Women in chemistry: their changing roles from alchemical times to the mid-twentieth century. Philadelphia: Chemical Heritage Foundation. pp. 28–31. ISBN 978-0941901277. Retrieved 2 March 2016.
- Datta, N. C. (2005). The story of chemistry. Hyderabad: Universities Press. pp. 247–250. ISBN 9788173715303. Retrieved 2 March 2016.
- Wikipedia https://en.wikipedia.org/wiki/Phlogiston_theory
Biography
Elizabeth Fulhame (fl. 1794) was an early British chemist who invented the concept of catalysis and discovered photoreduction. She was described as ‘the first solo woman researcher of modern chemistry’.
Although she only published one text, she describes catalysis as a process at length in her 1794 book An Essay On Combustion with a View to a New Art of Dying and Painting, wherein the Phlogistic and Antiphlogistic Hypotheses are Proved Erroneous. The book relates in painstaking detail her experiments with oxidation-reduction reactions, and the conclusions she draws regarding phlogiston theory, in which she disagrees with both the Phlogistians and Antiphlogistians.
In 1798, the book was translated into German by Augustin Gottfried Ludwig Lentin as Versuche über die Wiederherstellung der Metalle durch Wasserstoffgas. In 1810, it was published in the United States, to much critical acclaim. That same year, Fulhame was made an honorary member of the Philadelphia Chemical Society. Thomas P. Smith applauded her work, stating that “Mrs. Fulhame has now laid such bold claims to chemistry that we can no longer deny the sex the privilege of participating in this science also.”
Personal life
Elizabeth Fulhame published under her married name, as Mrs. Fulhame. She was married to Thomas Fulhame, an Irish-born physician who had attended the University of Edinburgh and studied puerperal fever as a student of Andrew Duncan (1744–1828). Dr Thomas Fulhame was listed in Edinburgh directories between 1784–1800 (Bristo Square in 1784, Bristo Street in 1794, at 9 Society 1799, in Brown’s Square 1800). She is believed by some to have been Scottish, but the evidence for this seems to be little more than that her husband studied in Edinburgh — on that basis Charles Darwin’s wife Emma could be claimed as Scottish, but she clearly was not. Sir Benjamin Thompson, Count Rumford, referred to her as “the ingenious and lively Mrs. Fulhame”, however this opinion may reflect the style of her book.
Work

Mrs. Fulhame’s work began with her interest in finding a way of staining cloth with heavy metals under the influence of light. She originally considered calling her work An Essay on the Art of making Cloths of Gold, Silver, and other Metals, by chymical processes, but considering the “imperfect state of the art”, decided to select a title reflecting the broader implications of her experiments.: viii–ix
“The possibility of making cloths of gold, silver, and other metals, by chymical processes, occurred to me in the year 1780: the project being mentioned to Doctor Fulhame, and some friends, was deemed improbable. However, after some time, I had the satisfaction of realizing the idea, in some degree, by experiment.”:
She was apparently encouraged to publish an account of her 14 years of research as a result of meeting Sir Joseph Priestley in 1793. Fulhame studied the experimental reduction of metallic salts in a variety of states (aqueous solution, dry state, and sometimes an ether or alcohol solution) by exposing them to the action of various reducing agents. The metal salts she examined included gold, silver, platinum, mercury, copper, and tin. As reducing agents, she experimented with hydrogen gas, phosphorus, potassium sulfide, hydrogen sulfide, phosphine, charcoal, and light. She discovered a number of chemical reactions by which metal salts could be reduced to pure metals. Rayner-Canham considers her most important contribution to chemistry to be the discovery that metals could be processed through aqueous chemical reduction at room temperature, as an alternative to smelting at high temperatures.
Her theoretical work on catalysis was “a major step in the history of chemistry”, predating both Jöns Jakob Berzelius and Eduard Buchner. She proposed, and demonstrated through experiment, that many oxidation reactions occur only in the presence of water, that they directly involve water, and that water is regenerated and is detectable at the end of the reaction. Further, she proposed “recognisably modern mechanisms” for those reactions, and may have been the first scientist to do so. The role of oxygen, as she describes it, differs significantly from other theories of the time. Based on her experiments, she disagreed with some of the conclusions of Antoine Lavoisier as well as with the phlogiston theorists that he critiqued. Her research could be seen as a precursor to the work of Jöns Jakob Berzelius, however Fulhame focused specifically on water rather than heavy metals.
Further, Eder, in 1905, and Schaaf consider her work on silver chemistry to be a landmark in the birth and early history of photography. Fulhame’s work on the role of light sensitive chemicals (silver salts) on fabric, predates Thomas Wedgwood’s more famous photogram trials of 1801. Fulhame did not, however, attempt to make “images” or representational shadow prints in the way Wedgwood did, but she did engage in photoreduction using light.
Reception
In addition to her book being republished in Germany and America, Fulhame’s experiments were reviewed in a French journal, and several British magazines, and were positively commented on by Sir Benjamin Thompson, Count Rumford, and Sir John Herschel.
According to the introduction of her book by her American editor in 1810, her work was lesser known than it could or should have been, adding that “the pride of science, revolted at the idea of being taught by a female”.
Fulhame says as much in her own preface to the work:
“But censure is perhaps inevitable: for some are so ignorant, that they grow sullen and silent, and are chilled with horror at the sight of anything that nears the semblance of learning, in whatever shape it may appear; and should be the spectre appear in the shape of a woman, the pangs which they suffer are truly dismal.”
Such a reaction, she says, was particularly acute amongst some who held esteemed positions, whom she described as having a ‘dictatorship in science’.
Fulhame published her experiments on reductions using water with metals in a book in the first place in order not to be “plagiarized.” She also describes her book as possibly serving as “a beacon to future mariners” (e.g. women) taking up scientific inquiries. Antoine Lavoisier was executed six months before the publication of her book and thus could not respond to her theory. Irish chemist William Higgins complained that she had ignored his work on the involvement of water in the rusting of iron, but magnanimously concluded “I read her book with great pleasure, and heartily wish that her laudible example may be followed by the rest of her sex.”
Fulhame’s work was largely forgotten by the end of the 19th century, but it was rediscovered by J. W. Mellor. In the 20th century, she was noted in Physics Today, as being the first to ‘systematically’ vary ‘her reaction conditions’ and to ‘generalise a whole class of reactions…. the reduction of metals’ and first to suggest an explanation for the situations where ‘water dissociated into its ionic form, facilitated the intermediate reaction steps, and was regenerated by the end of the metal reduction.’
See also
References
- Rayner-Canham, Marelene; Rayner-Canham, Geoffrey (2001). Women in chemistry : their changing roles from alchemical times to the mid-twentieth century. Philadelphia: Chemical Heritage Foundation. pp. 28–31. ISBN 978-0941901277. Retrieved 2 March 2016.
- “The mysterious case of Elizabeth Fulhame, a chemist and true pioneer of science”. The National. 31 January 2023. Retrieved 6 February 2023.
- Burwick, Frederick; Goslee, Nancy Moore; Hoeveler, Diane Long, eds. (2012). The encyclopedia of Romantic literature. Chichester, West Sussex [England]: Wiley-Blackwell. ISBN 9781405188104. Archived from the original on 7 March 2016. Retrieved 2 March 2016.
- Ogilvie, Marilyn Bailey (1986). Women in Science: Antiquity through the Nineteenth Century (4th print. ed.). Cambridge, Mass.: MIT Press. pp. 28–31. ISBN 978-0-262-65038-0.
- “Celebrating Scottish women of science”. National Library of Scotland. Archived from the original on 15 July 2017. Retrieved 20 June 2017.
- “Elizabeth Fulhame”. The Human Touch of Chemistry. Archived from the original on 3 March 2016. Retrieved 2 March 2016.
- Cornish-Bowden, Athel (2012). Fundamentals of enzyme kinetics. Weinheim: Wiley-VCH. ISBN 9783527665495. Retrieved 2 March 2016.
- Fulhame, T. (1784). Dissertatio de febre puerperarum. Academiæ Edinburgenæ, facultatis medicæ; pro gradu doctoris. Thomas Fulhame, M.A. hibernus. Ad diem 13. Septemb. Edinburgi: Apud Balfour et Smellie, academiae typographos. University of Edinburgh, Centre for Research Collections.
- “(456) – Towns > Edinburgh > 1773–1776, 1784–1785 – Williamson’s directory for the City of Edinburgh, Canongate, Leith and suburbs > 1784–85 – Scottish Directories – National Library of Scotland”. digital.nls.uk. Retrieved 10 February 2020.
- “(88) – Towns > Edinburgh > 1794–1795 – Directory for Edinburgh Leith Mussleburgh and Dalkeith – Scottish Directories – National Library of Scotland”. digital.nls.uk. Retrieved 10 February 2020.
- “(146) – Towns > Edinburgh > 1799–1800 – Edinburgh and Leith directory, to July 1800 – Scottish Directories – National Library of Scotland”. digital.nls.uk. Retrieved 10 February 2020.
- “(109) – Towns > Edinburgh > 1800–01 – Edinburgh and Leith directory to July 1801 – Scottish Directories – National Library of Scotland”. digital.nls.uk. Retrieved 10 February 2020.
- Ewan, Elizabeth L., ed. (2006). The biographical dictionary of Scottish women : from the earliest times to 2004. Edinburgh: Edinburgh Univ. Press. p. 130. ISBN 9780748617135. Retrieved 2 March 2016.
- Rumford, Benjamin, Graf von (1875). The complete works of Count Rumford. Vol. 4. Boston: American Academy of Arts and Sciences. p. 84. Retrieved 2 March 2016.
- Fulhame, Elizabeth (1794). An essay on combustion, with a view to a new art of dying and painting. Wherein the phlogistic and antiphlogistic hypotheses are proven erroneous. London: Printed for the author, by J. Cooper. Retrieved 2 March 2016.|
- Batchen, Geoffrey (1997). Burning with desire : the conception of photography (First MIT Press paperback ed.). Cambridge, Mass.: MIT Press. p. 28. ISBN 9780262024273.
- Davenport, Derek; Ireland, Kathleen (1989). “The Ingenious, Lively and Celebrated Mrs. Fulhame and the Dyer’s Hand” (PDF). Bulletin for the History of Chemistry (5): 37–42. Retrieved 2 March 2016.
- Laidler, Keith J.; Cornish-Bowden, Athel (1997). “”Elizabeth Fulhame and the discovery of catalysis: 100 years before Buchner” (PDF). In Cornish-Bowden, Athel (ed.). New beer in an old bottle : Eduard Buchner and the growth of biochemical knowledge. Valencia: Universitat de Valencia. pp. 123–126. ISBN 9788437033280. Archived from the original (PDF) on 23 January 2015. Retrieved 2 March 2016.
- Datta, N. C. (2005). The story of chemistry. Hyderabad: Universities Press. pp. 247–250. ISBN 9788173715303. Retrieved 2 March 2016.
- Eder, Josef Maria (1905). Geschichte der Photographie (in German) (3rd ed.). Halle: Wilhelm Knapp. OCLC 494529056.
- Laidler, Keith J. (1993). To Light such a Candle. Oxford University Press. pp. 68–69.
- Schaaf, Larry J. (1990). “The first fifty years of British photography, 1794–1844”. In Pritchard, Michael (ed.). Technology and art: the birth and early years of photography: the proceedings of the Royal Photographic Historical Group conference 1–3 September 1989. Bath: RPS Historical Group. pp. 9–18. ISBN 9780951532201.
- Schaaf, Larry J. (1992). Out of the shadows: Herschel, Talbot, & the invention of photography. New Haven: Yale University Press. pp. 23–25. ISBN 9780300057058.
- “The Woman Behind Photography”. Sotheby’s Institute of Art. Retrieved 8 March 2016.
- Fulhame, Elizabeth (1810). An essay on combustion, with a view to a new art of dying and painting : wherein the phlogistic and antiphlogistic hypotheses are proved erroneous. Philadelphia: Printed and sold by James Humphreys, Corner of Second and Walnut-streets. p. iv. Retrieved 20 June 2017.
- Linker, Jessica C. (April 2015). “The Pride of Science: Women and the politics of inclusion in 19th century Philadelphia” (PDF). Pennsylvania Legacies. 15 (1): 6–11. doi:10.5215/pennlega.15.1.0006. Retrieved 2 March 2016.
- Mellor, J. W. (1903). “History of the water problem (Mrs Fulhame’s theory of catalysis)”. Journal of Physical Chemistry. 7 (8): 557–567. doi:10.1021/j150053a001.
- Wikipedia https://en.wikipedia.org/wiki/Elizabeth_Fulhame




